Global Immunodiagnostics Market
Market Size in USD Billion
CAGR :
% 
 USD
21.46 Billion
USD
56.26 Billion
2024
2032
USD
21.46 Billion
USD
56.26 Billion
2024
2032
| 2025 –2032 | |
| USD 21.46 Billion | |
| USD 56.26 Billion | |
|
|
|
|
Immunodiagnostics Market Size
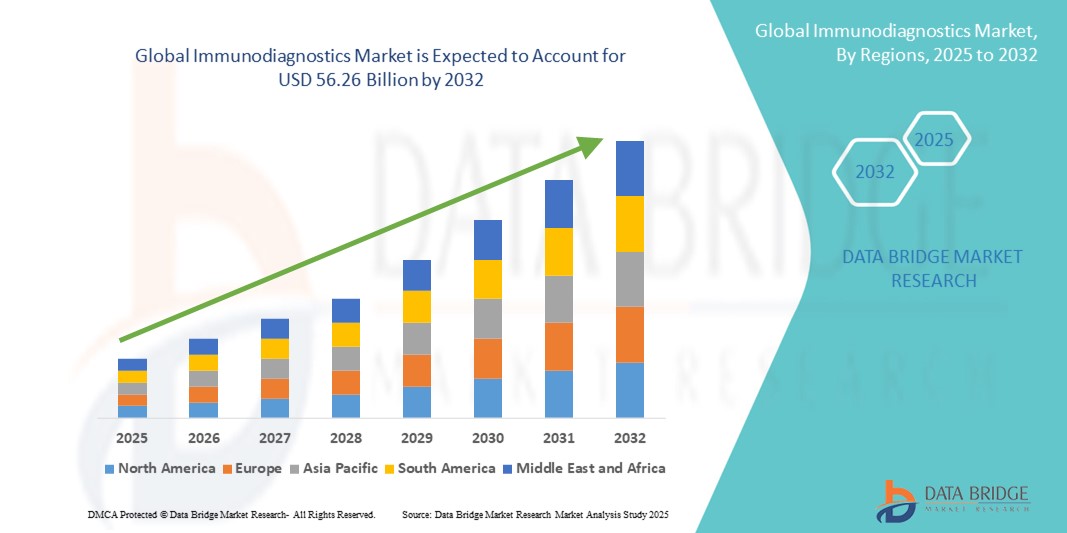
- The global immunodiagnostics market size was valued at USD 21.46 billion in 2024 and is expected to reach USD 56.26 billion by 2032, at a CAGR of 12.80% during the forecast period
- The market growth is largely fueled by the increasing prevalence of infectious diseases, cancer, and autoimmune disorders, which is driving higher adoption of immunodiagnostic techniques for accurate and early disease detection. Continuous technological advancements in assay platforms and diagnostic tools are further supporting market expansion by improving sensitivity, specificity, and turnaround time
- Furthermore, rising demand for rapid, reliable, and user-friendly diagnostic solutions across hospitals, diagnostic laboratories, and point-of-care settings is establishing immunodiagnostics as a preferred diagnostic method. These converging factors are accelerating the uptake of immunodiagnostic solutions, thereby significantly boosting the industry’s growth
Immunodiagnostics Market Analysis
- Immunodiagnostics, encompassing techniques and devices for detecting diseases through immune response markers, are increasingly vital components of modern clinical diagnostics supporting early disease detection, personalized medicine, and monitoring in both hospital and laboratory settings
- The escalating demand for immunodiagnostic solutions is primarily fueled by rising prevalence of chronic diseases, growing awareness of early diagnosis benefits, advancements in diagnostic technologies, and expanding application in infectious diseases and oncology
- North America dominated the immunodiagnostics market with the largest revenue share of 41.2% in 2024, characterized by advanced healthcare infrastructure, significant research investment, high disposable incomes, and a strong presence of key industry players. The U.S. experienced substantial growth driven by innovations in immunoassay platforms, molecular diagnostics integration, and expanding applications across hospitals and diagnostic laboratories
- Asia-Pacific is expected to be the fastest growing region in the Immunodiagnostics market during the forecast period, driven by increasing healthcare access, rising incidence of infectious and lifestyle diseases, expanding diagnostics infrastructure, and rapidly growing investments in healthcare technologies
- The Reagents segment dominated the immunodiagnostics market with the largest revenue share of 62.4% in 2024, driven by their recurring demand and indispensable role in every immunoassay procedure. Reagents such as antibodies, antigens, substrates, and buffers form the backbone of diagnostic tests, ensuring both sensitivity and accuracy
Report Scope and Immunodiagnostics Market Segmentation
|
Attributes |
Immunodiagnostics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Immunodiagnostics Market Trends
Enhanced Convenience Through Advanced Diagnostic Technologies
- A significant and accelerating trend in the global Immunodiagnostics market is the integration of advanced diagnostic platforms with automation and digital health technologies. This evolution is enhancing the accuracy, speed, and convenience of diagnostic testing in both clinical and research settings
- For instance, next-generation immunoassay platforms now allow rapid, high-throughput testing with improved sensitivity and specificity, significantly reducing turnaround times for results and enabling faster clinical decision-making
- The adoption of advanced immunodiagnostic technologies is also being supported by innovations in assay design, such as multiplexing capabilities that allow simultaneous testing for multiple conditions in a single sample. This reduces cost, improves efficiency, and optimizes patient management
- Automation within immunodiagnostics laboratories is driving operational convenience by minimizing manual errors, streamlining workflow, and enabling laboratories to handle growing test volumes with greater efficiency
- The integration of immunodiagnostics with digital health platforms further enhances convenience for healthcare providers and patients, enabling seamless data sharing, remote monitoring, and improved coordination of care across health systems
- This trend towards more intelligent, automated, and interconnected diagnostic solutions is reshaping expectations in healthcare, with leading companies developing platforms that provide faster results, improved accuracy, and greater accessibility across hospitals, laboratories, and point-of-care settings
Immunodiagnostics Market Dynamics
Driver
Growing Need Due to Rising Prevalence of Chronic and Infectious Diseases
- The increasing global burden of chronic illnesses such as cancer, cardiovascular disorders, and autoimmune diseases, along with the growing incidence of infectious diseases, is a significant driver for the heightened demand for Immunodiagnostics solutions
- For instance, in April 2024, Abbott announced an expansion of its immunoassay test portfolio to address the rising demand for rapid and accurate infectious disease diagnostics. Such strategies by key companies are expected to drive the Immunodiagnostics industry growth during the forecast period
- As healthcare systems worldwide face mounting pressure to deliver accurate, timely, and cost-efficient diagnostics, immunodiagnostics provides advanced features such as high sensitivity, specificity, and the ability to detect early-stage diseases — offering a compelling alternative to traditional diagnostic methods
- Furthermore, the growing adoption of preventive healthcare practices and the rising demand for personalized medicine are making immunodiagnostics an integral component of modern healthcare systems, ensuring faster clinical decision-making and better patient outcomes
- The convenience of automated immunodiagnostic platforms, high-throughput testing capabilities, and integration with digital health systems are key factors propelling adoption across hospitals, laboratories, and point-of-care facilities. The increasing availability of user-friendly testing kits and advanced immunoassay analyzers further contributes to global market growth
Restraint/Challenge
Concerns Regarding High Costs and Regulatory Complexities
- Despite strong growth prospects, the high costs associated with advanced immunodiagnostic instruments and assays pose a significant challenge to broader adoption, particularly in low- and middle-income countries with limited healthcare budgets
- For instance, certain advanced chemiluminescence and multiplex immunoassay systems require substantial upfront investment, making them less accessible to smaller diagnostic laboratories. This cost factor can limit the expansion of immunodiagnostics testing in resource-constrained settings
- Addressing these financial barriers through affordable test kits, government reimbursement initiatives, and collaborations with non-profit organizations is crucial for enabling wider adoption. Leading companies such as Roche and Siemens are focusing on scalable solutions to serve both developed and emerging markets
- In addition, regulatory complexities surrounding the approval of new diagnostic assays can delay market entry and adoption. Stringent requirements for clinical validation and compliance increase the time and cost for companies developing innovative solutions
- While regulatory oversight ensures safety and accuracy, it can also act as a restraint for smaller players. Overcoming these challenges through harmonized approval frameworks, strategic partnerships, and investment in cost-effective platforms will be vital for sustained market growth
Immunodiagnostics Market Scope
The market is segmented on the basis of Product, Technology, Application, and End User.
- By Product
On the basis of product, the immunodiagnostics market is segmented into reagents, instruments, and software and services. The Reagents segment dominated the market with the largest revenue share of 62.4% in 2024, driven by their recurring demand and indispensable role in every immunoassay procedure. Reagents such as antibodies, antigens, substrates, and buffers form the backbone of diagnostic tests, ensuring both sensitivity and accuracy. Their continuous consumption across hospitals, clinical laboratories, and research institutions guarantees sustained revenues compared to one-time instrument purchases. In addition, increasing demand for advanced reagents in high-sensitivity and point-of-care assays, coupled with innovations in biomarker-based tests, has reinforced their leadership. The growing use of automated immunoassay platforms globally also directly fuels reagent consumption, strengthening the segment’s dominance.
The Software and Services segment is projected to witness the fastest CAGR of 12.8% from 2025 to 2032, owing to the rising integration of digital platforms, laboratory information management systems (LIMS), and real-time analytics in diagnostic workflows. Healthcare providers increasingly rely on advanced software solutions to interpret large datasets, automate workflows, and meet stringent regulatory compliance. Service offerings such as system training, assay validation, and technical maintenance are becoming essential for enhancing laboratory performance and efficiency. Growing emphasis on interoperability across diagnostic platforms, combined with the rising demand for remote monitoring and reporting, further propels the adoption of software and service solutions in immunodiagnostics worldwide.
- By Technology
On the basis of technology, the immunodiagnostics market is segmented into enzyme-linked immunosorbent assay (ELISA), Chemiluminescence Immunoassay (CLIA), Fluorescent Immunoassay, Radioimmunoassay, Rapid Test, and Others. The Enzyme-Linked Immunosorbent Assay (ELISA) segment held the largest revenue share of 38.7% in 2024, driven by its long-standing role as a gold standard for diagnostic testing. ELISA’s versatility, cost-effectiveness, and ability to handle high sample volumes make it a preferred choice for detecting infectious diseases, autoimmune disorders, and allergens. Its reliability in both clinical diagnostics and research ensures consistent adoption across global laboratories. Moreover, the widespread availability of standardized ELISA kits and its compatibility with routine testing workflows enhance its strong market presence. Emerging economies are also heavily dependent on ELISA due to its affordability and proven diagnostic accuracy, cementing its leadership.
The Chemiluminescence Immunoassay (CLIA) segment is anticipated to witness the fastest CAGR of 13.6% from 2025 to 2032, owing to its superior sensitivity, accuracy, and automation capabilities. CLIA technology is increasingly adopted in hospitals and diagnostic centers for high-throughput testing of infectious diseases, cardiac markers, and oncology diagnostics. Its shorter turnaround times and wide testing menus make it highly attractive for advanced healthcare facilities. Continuous innovations in CLIA analyzers and reagent kits further enhance its diagnostic efficiency. With the growing shift toward precision diagnostics and faster clinical decision-making, CLIA is projected to see significant expansion across both developed and emerging markets during the forecast period.
- By Application
On the basis of application, the immunodiagnostics market is segmented into infectious diseases, oncology and endocrinology, hepatitis and retrovirus, bone and mineral, autoimmunity, cardiac biomarker, and others. The Infectious Diseases segment dominated the market with the largest revenue share of 45.1% in 2024, driven by the global prevalence of viral and bacterial infections. Immunodiagnostics play a vital role in the early detection of conditions such as HIV, tuberculosis, hepatitis, influenza, and COVID-19. Widespread government initiatives, vaccination drives, and routine screening programs continue to fuel the demand for infectious disease immunoassays. The segment also benefits from innovations in rapid immunoassays, point-of-care testing devices, and large-scale diagnostic programs in both developed and developing regions. This sustained demand ensures the segment’s continued market leadership.
The Oncology and Endocrinology segment is projected to expand at the fastest CAGR of 14.2% from 2025 to 2032, supported by rising focus on early cancer detection and monitoring of hormone-related disorders. Immunodiagnostic tests for tumor markers such as PSA, CA-125, and HER2 are gaining adoption in personalized medicine and therapy monitoring. In endocrinology, tests for thyroid dysfunction and diabetes management are witnessing growing importance due to increasing disease prevalence. The integration of immunodiagnostics in precision medicine and biomarker-driven drug development further accelerates demand. This segment is poised for significant growth as cancer and endocrine disorders continue to expand globally, requiring accurate, efficient, and sensitive diagnostic solutions.
- By End User
On the basis of end user, the immunodiagnostics market is segmented into clinical laboratories, hospitals, academic and research centers, Pharmaceutical and Biotechnology Industry, and Others. The Clinical Laboratories segment accounted for the largest market share of 49.6% in 2024, supported by their high testing volumes and established infrastructure. Clinical laboratories serve as central hubs for routine and specialized diagnostic testing, offering economies of scale and cost efficiencies. Their access to advanced immunoassay platforms, trained professionals, and established sample-handling protocols ensures accurate and timely results. Strong collaborations with healthcare providers and government health programs further reinforce the demand for immunodiagnostics in this segment. The growing need for regular health monitoring and large-scale population testing contributes to clinical laboratories’ continued dominance.
The Pharmaceutical and Biotechnology Industry segment is anticipated to register the fastest CAGR of 13.9% from 2025 to 2032, driven by the rising role of immunodiagnostics in drug discovery, vaccine development, and biomarker validation. Immunoassays are increasingly used in therapeutic monitoring, companion diagnostics, and clinical trials for biologics and immunotherapies. The growing demand for personalized medicine and targeted therapies has further expanded the application scope of immunodiagnostics within this industry. Substantial R&D investments in precision drug development, coupled with the adoption of advanced diagnostic technologies, position this segment for rapid and sustained growth throughout the forecast period.
Immunodiagnostics Market Regional Analysis
- North America dominated the immunodiagnostics market with the largest revenue share of 41.2% in 2024, reflecting the region’s advanced healthcare infrastructure, strong investment in research and development, and the presence of major industry players
- The region is characterized by early adoption of innovative immunodiagnostic platforms, widespread access to healthcare services, and a focus on preventive medicine. Rising prevalence of chronic illnesses, infectious diseases, and lifestyle-related conditions has further increased the demand for timely diagnostic testing
- High disposable incomes and supportive reimbursement frameworks also strengthen market adoption, making North America a key hub for immunodiagnostics innovation and deployment
U.S. Immunodiagnostics Market Insight
The U.S. immunodiagnostics market held the largest share within North America in 2024, driven by rapid advancements in immunoassay platforms, integration of molecular diagnostics, and expanding diagnostic applications across hospitals, clinical laboratories, and research institutions. The country continues to witness growing adoption of companion diagnostics, precision medicine, and automated testing platforms that improve efficiency and accuracy. Increasing disease burden, particularly in oncology and infectious diseases, combined with strong FDA approvals and funding for research, further accelerates growth. Moreover, the U.S. benefits from an innovation-driven ecosystem that fosters collaborations between biotechnology firms, healthcare providers, and academic research centers, ensuring strong future demand.
Europe Immunodiagnostics Market Insight
The Europe Immunodiagnostics market is projected to expand at a substantial CAGR during the forecast period, supported by its well-developed healthcare infrastructure, government funding for diagnostic research, and a strong regulatory framework ensuring high testing standards. Increasing cancer incidence, infectious disease outbreaks, and the rising prevalence of endocrine disorders have created significant demand for advanced immunodiagnostic tools. European consumers and healthcare systems also emphasize accurate and rapid disease detection, driving the integration of immunodiagnostics into both public and private healthcare facilities. Additionally, growing collaborations between diagnostic companies and universities are fostering innovation, further strengthening Europe’s position in the global market.
U.K. Immunodiagnostics Market Insight
The U.K. immunodiagnostics market is expected to grow at a noteworthy CAGR, supported by rising demand for advanced testing in hospitals, clinics, and research institutions. The country’s focus on healthcare modernization and diagnostic innovation is driving strong adoption of immunoassays, particularly in oncology and infectious diseases. Increased public awareness about preventive healthcare, combined with strong investments in the digitalization of healthcare services, is supporting greater accessibility to diagnostic solutions. The U.K.’s robust pharmaceutical and biotechnology sector also contributes to partnerships and research, boosting the development and deployment of new immunodiagnostic assays.
Germany Immunodiagnostics Market Insight
The Germany immunodiagnostics market is anticipated to expand steadily, fueled by the country’s highly developed healthcare system, strong focus on technological innovation, and emphasis on early disease detection. Demand for advanced immunodiagnostic tools is increasing in oncology, cardiology, and infectious disease testing, supported by government initiatives that encourage research and adoption of high-precision diagnostic solutions. Germany’s focus on sustainability and eco-conscious healthcare technologies also plays a role in driving innovation in diagnostic instruments and assays. Additionally, the country’s extensive network of hospitals, laboratories, and academic research centers continues to accelerate adoption of immunodiagnostic platforms.
Asia-Pacific Immunodiagnostics Market Insight
The Asia-Pacific immunodiagnostics market is projected to record the fastest CAGR during 2025–2032, driven by rapid healthcare infrastructure development, rising disposable incomes, and increasing demand for advanced diagnostics. The growing incidence of infectious diseases, lifestyle-related disorders, and cancer across the region has led to heightened demand for early detection and precision medicine. Countries such as China, Japan, and India are making significant investments in diagnostic technologies, supported by government initiatives promoting healthcare modernization and digitalization. The availability of affordable testing solutions and strong local manufacturing capabilities are also expanding access to immunodiagnostics across both urban and rural populations, making APAC a dynamic and fast-evolving market.
Japan Immunodiagnostics Market Insight
The Japan immunodiagnostics market is gaining momentum, supported by the country’s advanced technological ecosystem, high standards of healthcare, and emphasis on innovative diagnostic solutions. With an aging population and increasing prevalence of cancer and infectious diseases, demand for accurate and rapid immunoassays is rising significantly. The integration of immunodiagnostics into personalized medicine strategies and digital health systems is a key growth driver. Moreover, Japan’s culture of innovation, combined with collaborations between universities, hospitals, and biotech companies, is fostering continuous advancements in immunodiagnostic research and adoption.
China Immunodiagnostics Market Insight
The China immunodiagnostics market accounted for the largest revenue share in Asia-Pacific in 2024, reflecting the country’s rapid urbanization, growing middle-class population, and strong government support for healthcare reform. China has emerged as one of the largest markets for diagnostic technologies, with immunodiagnostics widely adopted for infectious disease management, oncology, and preventive healthcare. The presence of strong domestic manufacturers, combined with partnerships with international players, has boosted the availability of both advanced and cost-effective testing solutions. Furthermore, the country’s national focus on healthcare digitalization and smart hospital initiatives is expected to further accelerate market expansion in the coming years.
Immunodiagnostics Market Share
The immunodiagnostics industry is primarily led by well-established companies, including:
- Omega Diagnostics Ltd (U.K.)
- Nexus-Dx (U.S.)
- ProtaGene Group (Germany)
- Quest Diagnostics Incorporated (U.S.)
- Seramun Diagnostica GmbH (Germany)
- Tecan Trading AG (Switzerland)
- Siemens (Germany)
- DiaSorin S.p.A. (Italy)
- Erba Mannheim (U.S.)
- Svar Life Science AB (Sweden)
- Exagen Inc. (U.S.)
- Abbott (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Danaher Corporation. (U.S.)
- Adaptive Biotechnologies (U.S.)
- QIAGEN (Netherlands)
- AESKU.GROUP GmbH (Germany)
Latest Developments in Global Immunodiagnostics Market
- In July 2021, DiaSorin S.p.A. completed the acquisition of Luminex Corporation, strengthening its immunodiagnostics portfolio with multiplexing technology and expanding its presence in the U.S. diagnostics market. The deal enabled DiaSorin to broaden its offering in specialty and molecular testing, enhancing its position in infectious disease and immunoassay diagnostics
- In May 2022, Quidel Corporation completed its acquisition of Ortho Clinical Diagnostics, creating QuidelOrtho, a global diagnostics powerhouse. This merger combined Ortho’s expertise in immunoassay and clinical chemistry with Quidel’s strength in point-of-care and rapid testing, allowing the new entity to serve hospital labs, blood banks, and physician offices with a broad testing portfolio
- In November 2022, Beckman Coulter Diagnostics received FDA 510(k) clearance for its DxI 9000 Access Immunoassay Analyzer together with the Access TSH assay. The analyzer introduced advanced throughput, improved sensitivity, and automation features designed to streamline laboratory workflows and meet growing test demands
- In January 2023, the FDA granted 510(k) clearance for Roche’s Elecsys Anti-HCV II immunoassay, which enables the qualitative detection of hepatitis C virus antibodies on cobas e analyzers. This approval supports earlier and more accurate detection of HCV infection, strengthening blood screening and diagnostic capabilities in the U.S.
- In May 2023, the FDA authorized Thermo Fisher Scientific’s B·R·A·H·M·S sFlt-1/PlGF immunoassay, the first test of its kind in the U.S. to aid in evaluating the risk of severe preeclampsia in pregnant women. This test offers physicians an evidence-based tool to improve maternal care and outcomes by identifying patients at higher risk for complications
- In July 2023, Siemens Healthineers received FDA clearance for its Atellica CI Analyzer, a mid-volume platform combining clinical chemistry and immunoassay testing. This launch expanded Siemens’ Atellica portfolio, enabling greater flexibility and efficiency for hospitals and diagnostic laboratories.
- In February 2024, the FDA approved Roche’s Elecsys Anti-HBc II immunoassay as a donor-screening test for antibodies to the hepatitis B core antigen. This marked a significant step toward improving blood safety in the U.S., supporting reliable screening of donated blood and plasma
- In April 2024, Roche announced that its Elecsys pTau217 blood test for Alzheimer’s disease received FDA Breakthrough Device Designation. This test measures phosphorylated tau in blood, representing a potential shift toward less invasive and earlier detection of Alzheimer’s compared to cerebrospinal fluid or PET imaging
- In April 2024, the FDA cleared Abbott’s i-STAT TBI blood test, which measures GFAP and UCH-L1 biomarkers to help evaluate traumatic brain injury (TBI) at or near the point of care. This innovation provides emergency departments with faster, lab-quality results to improve patient triage
- In May 2025, the FDA approved the first Alzheimer’s disease blood test in the U.S. — the Lumipulse G pTau217/β-Amyloid 1-42 Plasma Ratio assay from Fujirebio. This test helps clinicians evaluate adults with cognitive symptoms for Alzheimer’s pathology, representing a breakthrough in accessible, blood-based neurodiagnostics
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.