Global Interferon Gamma Release Assays Igras Market
Market Size in USD Million
CAGR :
% 
 USD
518.27 Million
USD
973.58 Million
2024
2032
USD
518.27 Million
USD
973.58 Million
2024
2032
| 2025 –2032 | |
| USD 518.27 Million | |
| USD 973.58 Million | |
|
|
|
|
Interferon-Gamma Release Assays (IGRAs) Market Size
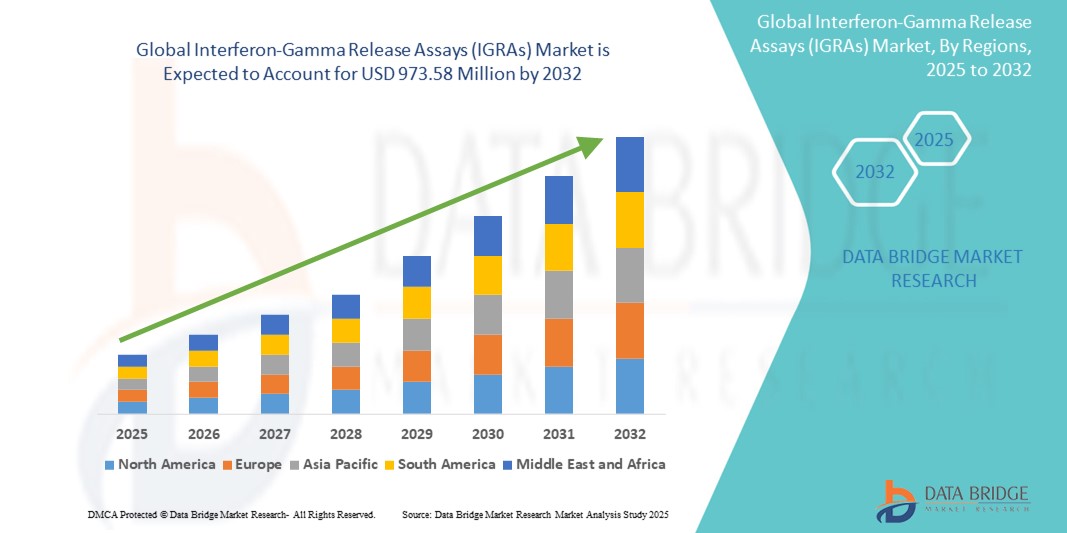
- The global interferon-gamma release assays (IGRAs) market size was valued at USD 518.27 million in 2024 and is expected to reach USD 973.58 million by 2032, at a CAGR of 8.20% during the forecast period
- This growth is driven by factors such as the rising global burden of tuberculosis, increased demand for accurate latent TB diagnosis, and growing awareness of preventive healthcare measures
Interferon-Gamma Release Assays (IGRAs) Market Analysis
- Interferon-gamma release assays (IGRAs) are blood tests used to detect latent tuberculosis infection (LTBI) by measuring the immune system's response to Mycobacterium tuberculosis-specific antigens. They serve as alternatives to the traditional Tuberculin Skin Test (TST)
- The demand for IGRAs is significantly driven by the rising global burden of tuberculosis, growing awareness regarding latent TB detection, and the need for accurate, quick diagnostics, especially in low- and middle-income countries
- North America is expected to dominate the interferon-gamma release assays (IGRAs)s market with a market share of 40.5%, due to widespread adoption of advanced TB diagnostics, strong public health infrastructure, and supportive reimbursement policies
- Asia-Pacific is expected to be the fastest growing region in the interferon-gamma release assays (IGRAs) market with a market share of 25.5%, during the forecast period due to increasing TB prevalence, rising healthcare investment, and greater awareness of latent TB screening
- Latent tuberculosis infection (LTBI) detection segment is expected to dominate the market with a market share of 60.5% due to its increasing global focus on early TB detection and prevention strategies
Report Scope and Interferon-Gamma Release Assays (IGRAs) Market Segmentation
|
Attributes |
Interferon-Gamma Release Assays (IGRAs) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Interferon-Gamma Release Assays (IGRAs) Market Trends
“Automation and Digital Integration in TB Diagnostics”
- A key trend in the IGRA market is the increasing adoption of automated and digitally integrated platforms to improve test accuracy, efficiency, and scalability in tuberculosis (TB) diagnostics
- These innovations streamline laboratory workflows by minimizing manual intervention, reducing the risk of human error, and enhancing consistency in IGRA test results
- For instance, platforms such as QIAGEN’s QuantiFERON-TB Gold Plus now support automation-compatible workflows with LIS connectivity and automated ELISA processing, significantly improving throughput in high-demand settings
- The integration of automation and digital tools is transforming TB testing, enabling broader screening initiatives, accelerating result turnaround times, and driving demand for advanced IGRA solutions globally
Interferon-Gamma Release Assays (IGRAs) Market Dynamics
Driver
“Rising Global Burden of Tuberculosis and Latent TB Infections”
- The increasing global burden of tuberculosis (TB), particularly in low- and middle-income countries, is a major driver of the IGRA market, as public health efforts intensify to identify and control both active and latent TB infections
- Latent TB infection (LTBI) affects an estimated 1.7 billion people globally, acting as a reservoir for future active TB cases if not properly diagnosed and treated
- IGRA tests offer greater specificity and convenience compared to traditional skin tests, making them ideal for large-scale screening in both high- and low-incidence settings
For instance,
- According to the World Health Organization (2023), TB remains one of the top infectious disease killers globally, with 10.6 million people falling ill and 1.3 million dying from TB in 2022. Early and accurate detection of latent infections is critical to achieving global TB elimination goals
- As global TB control programs expand and adopt newer diagnostic technologies, demand for Interferon-Gamma Release Assays continues to grow substantially
Opportunity
“Integration of Digital Health and AI Technologies in TB Diagnostics”
- The integration of digital health platforms and AI-based data analytics into IGRA testing workflows presents a major opportunity to enhance TB detection, reporting, and monitoring on a global scale
- AI algorithms can support clinical decision-making by analyzing large datasets from IGRA results, patient demographics, and comorbidities to predict TB risk and optimize treatment strategies
- In addition, digital tools such as mobile-connected diagnostic platforms can facilitate remote testing, real-time data sharing, and improved disease surveillance, particularly in resource-limited settings
For instance,
- In November 2024, the Stop TB Partnership highlighted the growing role of AI and digital connectivity in strengthening TB screening and diagnosis, noting pilot programs in India and South Africa that leverage AI-powered platforms for real-time IGRA test result interpretation and TB case tracking
- By enabling faster, more accurate, and more accessible testing, the integration of digital and AI technologies in IGRA diagnostics can significantly improve public health response and help meet global TB elimination targets
Restraint/Challenge
“High Test Costs and Limited Accessibility in Low-Income Regions”
- The relatively high cost of IGRA testing compared to traditional Tuberculin Skin Tests (TST) presents a significant barrier to widespread adoption, particularly in low- and middle-income countries where TB burden is highest
- IGRA tests require specialized laboratory equipment, trained personnel, and sometimes automation systems, making implementation more expensive and less feasible for rural or under-resourced healthcare settings
- This economic disparity can lead to continued reliance on less accurate methods, reducing the overall effectiveness of TB detection and control programs in high-need areas
For instance,
- According to a report by the Stop TB Partnership (2024), cost per IGRA test can range from USD 30 to USD 60, compared to under USD 5 for TSTs, making IGRAs financially out of reach for many national TB programs without international funding support
- Consequently, the high cost of IGRA testing limits market penetration in developing regions, contributing to inequities in diagnostic access and slowing the global effort to eliminate tuberculosis
Interferon-Gamma Release Assays (IGRAs) Market Scope
The market is segmented on the basis of product type, application, and end user
|
Segmentation |
Sub-Segmentation |
|
By Product type |
|
|
By Application |
|
|
By End User |
|
In 2025, the latent tuberculosis infection (LTBI) segment is projected to dominate the market with a largest share in application segment
The latent tuberculosis infection (LTBI) setection segment is expected to dominate the interferon-gamma release assays (IGRAs) market with the largest share of 60.5% due to its increasing global focus on early TB detection and prevention strategies. The growing awareness of LTBI's role in progressing to active tuberculosis and the rising demand for accurate, rapid diagnostic tools drive this dominance. In addition, government initiatives and public health programs targeting TB elimination further enhance the adoption of LTBI detection methods
The QuantiFERON-TB Gold Plus (QFT-Plus) is expected to account for the largest share during the forecast period in product type market
In 2025, the QuantiFERON-TB Gold Plus (QFT-Plus) segment is expected to dominate the market with the largest share of 30.5% in 2025 due to its high accuracy, reliability, and ease of use compared to traditional Tuberculin Skin Tests (TST). Its ability to provide results in a shorter time frame, coupled with its widespread adoption in clinical settings, contributes significantly to its dominance. In addition, the growing prevalence of latent tuberculosis and the shift toward more advanced diagnostic tools support its continued market leadership
Interferon-Gamma Release Assays (IGRAs) Market Regional Analysis
“North America Holds the Largest Share in the Interferon-Gamma Release Assays (IGRAs) Market”
- North America dominates the interferon-gamma release assays (IGRAs) market with a market share of estimated 40.5%, driven, by widespread adoption of advanced TB diagnostics, strong public health infrastructure, and supportive reimbursement policies
- U.S. holds a market share of 35.5%, due to high testing volumes, robust healthcare spending, and the presence of major players such as QIAGEN and Oxford Immunotec
- Government initiatives such as the CDC’s TB control programs and early adoption of technologies such as QuantiFERON-TB Gold Plus further contribute to the region’s leadership in this market
- In addition, growing immigration from high-TB-burden countries and mandatory screening policies are fueling demand for accurate and efficient TB diagnostics in the region
“Asia-Pacific is Projected to Register the Highest CAGR in the Interferon-Gamma Release Assays (IGRAs) Market”
- Asia-Pacific is expected to witness the highest growth rate in the interferon-gamma release assays (IGRAs) market with a market share of 25.5%, driven by increasing TB prevalence, rising healthcare investment, and greater awareness of latent TB screening
- Countries such as India, China, and Indonesia are key contributors due to large at-risk populations and national TB eradication programs that increasingly incorporate IGRAs into diagnostic strategies
- Japan’s emphasis on early TB detection through advanced diagnostics and China’s expanding public health surveillance systems make these countries important growth hubs for IGRA-based testing
- India is projected to register the highest CAGR in the Interferon-Gamma Release Assays (IGRAs) market, driven by government initiatives such as the National Strategic Plan for TB Elimination and the growing availability of automated IGRA platforms across public and private labs
Interferon-Gamma Release Assays (IGRAs) Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- QIAGEN (Netherlands)
- Revvity (U.S.)
- Bio-Techne (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Bio-Rad Laboratories, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Abbott (U.S.)
- Siemens Healthineers AG (Germany)
- BD (U.S.)
- EUROIMMUN Medizinische Labordiagnostika AG (Germany)
- Enzo Biochem Inc. (U.S.)
- RayBiotech, Inc. (U.S.)
- MyBioSource, Inc. (U.S.)
- Creative Diagnostics (U.S.)
- Sino Biological, Inc. (China)
- arigo Biolaboratories Corp. (Taiwan)
- LifeSpan BioSciences, Inc (U.S.)
- Abbexa (U.K.)
- Biomatik (Canada)
Latest Developments in Global Interferon-Gamma Release Assays (IGRAs) Market
- In April 2025, Revvity, Inc. received FDA approval for its Auto-Pure 2400 liquid handling platform integrated with the T-SPOT.TB test. This automation enhances laboratory workflows by processing up to 24 samples per run in under 3.5 hours, aiming to improve latent tuberculosis detection efficiency
- In February 2025, QIAGEN secured a favorable court decision from the German Federal Patent Court, reaffirming the validity of a key patent protecting innovations in its QuantiFERON-TB Gold Plus technology. This legal victory strengthens QIAGEN's intellectual property rights in the IGRA market
- In June 2024, The American Academy of Pediatrics updated its guidelines to recommend the use of IGRA tests, such as QIAGEN's QuantiFERON-TB Gold Plus, for screening latent tuberculosis infections in at-risk children of all ages. This endorsement is expected to increase the adoption of IGRAs in pediatric population
- In August 2024, The World Health Organization issued a public call for data to inform an update of its guidance on the use of IGRAs for detecting tuberculosis infection. This initiative aims to assess new IGRA technologies and their role in global TB control strategies
- In January 2022, the World Health Organization (WHO) announces that current WHO recommendations for the use of interferon-gamma release assays (IGRA) are also valid for Beijing Wantai’s TB-IGRA and Qiagen QuantiFERON-TB Gold Plus products. This expands the range of tests available to detect TB infection
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.