Global Johanson Blizzard Syndrome Market
Market Size in USD Billion
CAGR :
% 
 USD
165.90 Billion
USD
239.56 Billion
2024
2032
USD
165.90 Billion
USD
239.56 Billion
2024
2032
| 2025 –2032 | |
| USD 165.90 Billion | |
| USD 239.56 Billion | |
|
|
|
|
Johanson Blizzard Syndrome Market Size
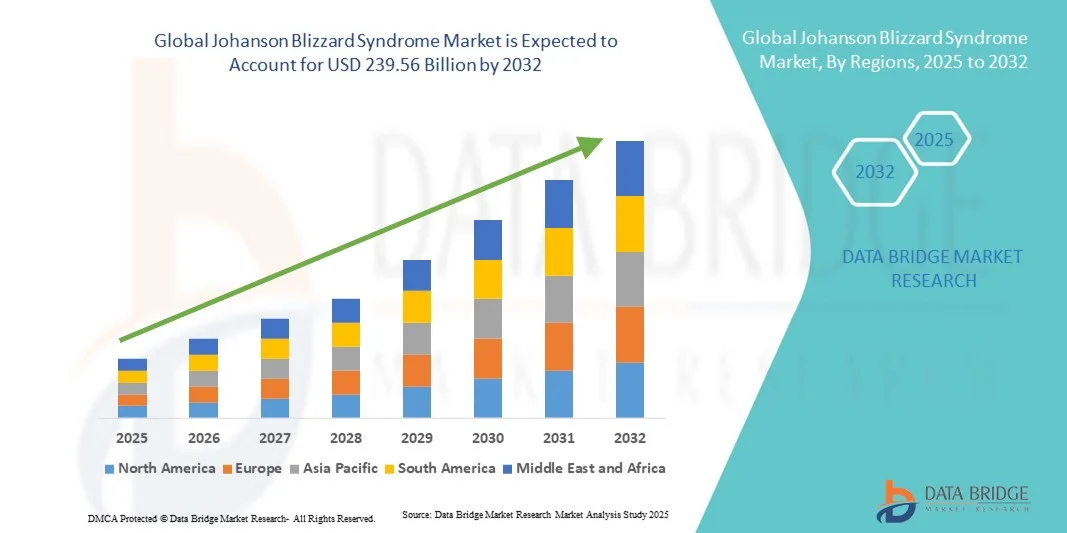
- The global Johanson Blizzard Syndrome market size was valued at USD 165.90 billion in 2024 and is expected to reach USD 239.56 billion by 2032, at a CAGR of 4.70% during the forecast period
- The market growth is largely fueled by increasing advancements in genetic testing, molecular diagnostics, and the rising awareness of rare congenital disorders, leading to improved detection and management of Johanson Blizzard Syndrome globally
- Furthermore, growing research initiatives, orphan drug designations, and collaborative efforts among academic and pharmaceutical organizations are enhancing treatment development and patient care, thereby accelerating the expansion of the Johanson Blizzard Syndrome market
Johanson Blizzard Syndrome Market Analysis
- Johanson Blizzard Syndrome (JBS), a rare congenital multisystem disorder caused by mutations in the UBR1 gene, is gaining increased clinical attention due to advancements in molecular diagnostics and genetic screening, which are enabling earlier and more accurate detection of the condition
- The rising demand for advanced diagnostic solutions and supportive care approaches is primarily fueled by growing awareness of rare genetic disorders, expanding research in genetic counseling, and improved healthcare access across both developed and developing regions
- North America dominated the Johanson Blizzard Syndrome market with the largest revenue share of 42.1% in 2024, supported by the presence of specialized research institutions, strong funding for rare disease programs, and widespread adoption of next-generation sequencing technologies
- Asia-Pacific is anticipated to be the fastest-growing region during the forecast period, driven by increasing investments in genomic research, rising healthcare expenditure, and the establishment of rare disease registries in countries such as Japan, China, and India
- The Pancreatic Insufficiency segment dominated the Johanson Blizzard Syndrome market with a market share of 46.8% in 2024, owing to its high prevalence among diagnosed patients and the growing focus on enzyme replacement therapy and nutritional management to improve patient outcomes
Report Scope and Johanson Blizzard Syndrome Market Segmentation
|
Attributes |
Johanson Blizzard Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Johanson Blizzard Syndrome Market Trends
Advancements in Genetic Testing and Molecular Diagnostics
- A significant and accelerating trend in the global Johanson Blizzard Syndrome (JBS) market is the growing application of advanced genetic sequencing technologies such as next-generation sequencing (NGS) and whole-exome sequencing (WES) for accurate identification of UBR1 gene mutations. This technological progress is improving diagnostic precision and enabling earlier detection
- For instance, laboratories specializing in rare genetic disorders have integrated comprehensive gene panels that include UBR1 testing, allowing for faster and more reliable confirmation of JBS in suspected cases. Similarly, clinical genomics platforms are now offering expanded testing capabilities for rare multisystem syndromes
- The integration of molecular diagnostics is also enabling more personalized patient management, allowing clinicians to identify genotype-phenotype correlations and tailor treatment approaches accordingly. For instance, improved molecular characterization of UBR1 variants is helping to refine disease prognosis and inform therapeutic strategies
- Furthermore, increasing collaboration between genetic testing companies, hospitals, and research institutions is enhancing data sharing and case identification, leading to the creation of global rare disease databases. These networks are fostering faster diagnosis and more effective clinical management.
- • This trend toward molecular-level precision and data-driven collaboration is fundamentally reshaping the rare disease diagnostics landscape. Consequently, companies are investing in developing more affordable and accessible genetic testing kits to support early detection of Johanson Blizzard Syndrome
- The demand for advanced diagnostic tools and genetic analysis platforms is growing rapidly across both developed and emerging economies, as healthcare providers prioritize early identification and intervention for rare congenital disorders
- The adoption of AI-powered bioinformatics tools for rare disease variant interpretation is increasing, improving diagnostic speed and accuracy for JBS by automating mutation analysis and phenotype mapping
- Expansion of tele-genetics and digital consultation platforms is helping bridge diagnostic gaps, enabling remote genetic counseling and second opinions for patients in underserved regions
Johanson Blizzard Syndrome Market Dynamics
Driver
Rising Research Initiatives and Orphan Drug Development
- The increasing focus on rare disease research and growing global efforts to develop orphan drugs are significant drivers propelling the Johanson Blizzard Syndrome market
- For instance, academic collaborations and nonprofit foundations are supporting genetic research programs that aim to uncover therapeutic pathways for JBS and similar multisystem disorders
- As governments and international organizations expand funding for rare genetic diseases, more resources are being directed toward diagnostic improvement, clinical registries, and treatment innovation
- Furthermore, the rise in public awareness campaigns and patient advocacy initiatives is increasing diagnosis rates and improving access to care for individuals affected by JBS worldwide
- The development of orphan drugs, potential gene therapies, and enzyme replacement treatments targeting the underlying genetic causes of the disorder are key factors driving market growth. The supportive regulatory environment for rare disease drug approvals further enhances this momentum
- Growing collaborations between biotech firms, genetic researchers, and healthcare providers are fostering innovation in treatment development and improving the global landscape for JBS management
- Increasing availability of government incentives and grants for orphan disease R&D is motivating pharmaceutical companies to explore novel treatment avenues for JBS
- Expansion of global rare disease registries and biobanking initiatives is supporting large-scale data collection, facilitating deeper understanding of disease mechanisms and treatment response patterns
Restraint/Challenge
Limited Clinical Awareness and Diagnostic Accessibility
- Limited awareness of Johanson Blizzard Syndrome among healthcare professionals and delayed clinical recognition remain key challenges restraining the market’s growth
- For instance, due to its rarity and overlapping symptoms with other pancreatic and developmental disorders, JBS is often misdiagnosed or diagnosed late, affecting timely intervention and care
- The scarcity of specialized diagnostic laboratories and limited access to advanced genetic testing technologies in developing regions further restrict accurate detection rates
- In addition, the high cost associated with genetic testing and limited insurance coverage for rare diseases can pose barriers to early diagnosis and treatment
- While research efforts are expanding, the small patient population size makes large-scale clinical trials challenging, slowing therapeutic advancements and commercial viability for pharmaceutical companies
- Overcoming these challenges through clinician education, international data sharing, and broader access to affordable diagnostic tools will be critical for improving early detection and enhancing patient outcomes in the Johanson Blizzard Syndrome market
- Lack of standardized clinical management protocols across healthcare systems hinders consistent treatment and long-term monitoring of JBS patients
- Limited patient registries and fragmented data reporting impede epidemiological research, making it difficult to assess disease prevalence and evaluate therapy effectiveness globally
Johanson Blizzard Syndrome Market Scope
The market is segmented on the basis of symptoms and related disorders.
- By Symptoms
On the basis of symptoms, the Johanson Blizzard Syndrome market is segmented into pancreatic insufficiency, tooth abnormalities, and characteristic shape of the nose. The pancreatic insufficiency segment dominated the market with the largest revenue share of 46.8% in 2024, driven by its high prevalence among patients diagnosed with Johanson Blizzard Syndrome (JBS). This symptom is a key clinical indicator due to exocrine pancreatic dysfunction, which results in malabsorption, growth retardation, and nutritional deficiencies. The growing adoption of enzyme replacement therapies (ERT) and nutritional support products has expanded clinical management options for this symptom. In addition, increasing awareness among pediatricians and gastroenterologists regarding genetic causes of pancreatic insufficiency has improved diagnostic accuracy. Continuous advances in biochemical testing and enzyme therapy optimization are expected to sustain dominance of this segment over the forecast period. The strong clinical relevance and direct link to disease severity make pancreatic insufficiency the most critical area of therapeutic and research focus within the JBS market.
The tooth abnormalities segment is anticipated to witness the fastest growth rate from 2025 to 2032, attributed to rising awareness of dental manifestations as an early diagnostic clue in suspected JBS cases. These abnormalities, including hypodontia, conical teeth, and delayed eruption, are gaining importance in multidisciplinary care involving dental specialists and geneticists. For instance, dental research collaborations have begun integrating oral phenotype mapping into genetic screening protocols for rare syndromes. Advancements in dental imaging, restorative techniques, and preventive care for genetically linked conditions are further driving segment growth. The inclusion of dental anomalies in rare disease registries and genetic counseling frameworks highlights their expanding diagnostic value. As precision medicine advances, early identification of dental indicators may significantly contribute to faster detection and improved patient outcomes in JBS.
- By Related Disorders
On the basis of related disorders, the Johanson Blizzard Syndrome market is segmented into cystic fibrosis, shwachman syndrome, and pearson marrow-pancreas syndrome. The cystic fibrosis segment dominated the related disorders category with the largest market revenue share of 44.8% in 2024, owing to its clinical overlap with pancreatic and respiratory dysfunctions observed in Johanson Blizzard Syndrome. The widespread availability of diagnostic infrastructure for cystic fibrosis, including sweat chloride testing and CFTR gene sequencing, indirectly benefits early screening for JBS through differential diagnosis. Increased physician awareness and genetic cross-testing between these disorders are strengthening diagnostic precision. Furthermore, ongoing research comparing pancreatic pathophysiology between cystic fibrosis and JBS is helping identify shared therapeutic opportunities. Collaboration between cystic fibrosis research foundations and rare disease programs is also driving innovation in enzyme replacement and nutritional interventions applicable to JBS management.
The Shwachman Syndrome segment is projected to record the fastest growth during the forecast period, driven by expanding clinical research and genetic comparison studies highlighting its phenotypic similarities to Johanson Blizzard Syndrome. Shwachman Syndrome shares overlapping symptoms such as exocrine pancreatic insufficiency, skeletal abnormalities, and hematologic complications, prompting clinicians to use parallel testing panels for accurate differentiation. For instance, advancements in next-generation sequencing have enabled dual detection of UBR1 and SBDS gene mutations, improving diagnostic efficiency. The growing establishment of international consortia studying bone marrow-pancreas disorders and genetic overlap between rare diseases is fueling awareness and cross-research initiatives. As diagnostic technologies evolve and multidisciplinary collaboration strengthens, the Shwachman Syndrome segment is expected to see accelerated market expansion supported by shared therapeutic approaches and genetic insights.
Johanson Blizzard Syndrome Market Regional Analysis
- North America dominated the Johanson Blizzard Syndrome market with the largest revenue share of 42.1% in 2024, supported by the presence of specialized research institutions, strong funding for rare disease programs, and widespread adoption of next-generation sequencing technologies
- Patients and healthcare providers in the region benefit from improved access to next-generation sequencing (NGS), molecular diagnostics, and genetic counseling services, enabling earlier detection and management of Johanson Blizzard Syndrome
- This widespread adoption of advanced diagnostic tools is further supported by high healthcare spending, government funding for orphan diseases, and strong collaborations between biotech companies and academic research centers, establishing North America as the leading hub for Johanson Blizzard Syndrome research and clinical advancements
U.S. Johanson Blizzard Syndrome Market Insight
The U.S. Johanson Blizzard Syndrome market captured the largest revenue share of 79% in 2024 within North America, driven by strong genetic research infrastructure and advanced diagnostic capabilities. Increasing utilization of next-generation sequencing (NGS) and comprehensive genetic counseling programs is enhancing early detection rates. The country’s robust presence of biotechnology firms and academic research centers focusing on rare diseases further propels innovation in diagnostics and potential therapeutic development. Moreover, the growing number of patient advocacy groups and government-supported orphan disease initiatives continues to strengthen awareness and patient care for Johanson Blizzard Syndrome across the U.S.
Europe Johanson Blizzard Syndrome Market Insight
The Europe Johanson Blizzard Syndrome market is projected to expand at a steady CAGR throughout the forecast period, fueled by the presence of established genetic testing facilities and collaborative rare disease research networks. Increasing investment in genomic medicine and cross-border initiatives such as the European Reference Networks (ERNs) are promoting faster diagnosis and improved patient management. The region’s healthcare systems emphasize early genetic screening and multidisciplinary treatment, which supports market expansion. In addition, the adoption of digital health platforms and the inclusion of rare disease diagnostics in public health policies are strengthening Europe’s leadership in rare disease research and management.
U.K. Johanson Blizzard Syndrome Market Insight
The U.K. Johanson Blizzard Syndrome market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by expanding national genomics programs and research funding for rare congenital disorders. The country’s commitment to precision medicine, particularly through the Genomics England initiative, enhances early identification of rare genetic mutations such as those causing JBS. Increasing awareness among clinicians, supported by National Health Service (NHS) guidelines on rare disease management, is improving patient diagnosis and care. Moreover, partnerships between universities, hospitals, and biotechnology firms are fostering innovation in genetic testing and therapeutic exploration.
Germany Johanson Blizzard Syndrome Market Insight
The Germany Johanson Blizzard Syndrome market is expected to expand at a considerable CAGR during the forecast period, supported by strong emphasis on genetic innovation, patient registries, and collaborative medical research. Germany’s advanced healthcare infrastructure and focus on integrating molecular diagnostics in routine clinical practice drive early and accurate detection of JBS. The growing participation of German research institutions in European rare disease consortia and increased investment in biobank data utilization further strengthen diagnostic development. In addition, public health initiatives promoting rare disease awareness are fostering better coordination between clinicians and genetic laboratories.
Asia-Pacific Johanson Blizzard Syndrome Market Insight
The Asia-Pacific Johanson Blizzard Syndrome market is poised to grow at the fastest CAGR of 23.8% from 2025 to 2032, fueled by expanding genomic research capabilities and improved healthcare accessibility in countries such as China, Japan, and India. The increasing establishment of regional rare disease registries, government-led genetic screening initiatives, and rising investments in medical technology are driving market growth. Moreover, collaborations between Asian research institutes and Western biotechnology companies are enhancing access to advanced diagnostic tools. The region’s growing patient awareness and integration of precision medicine programs are contributing significantly to early diagnosis and effective management of JBS.
Japan Johanson Blizzard Syndrome Market Insight
The Japan Johanson Blizzard Syndrome market is gaining traction due to the country’s strong focus on advanced genetics, precision medicine, and healthcare innovation. The presence of cutting-edge molecular diagnostics and government-supported rare disease programs such as the Nanbyo Research Initiative promotes early detection and research advancement. For instance, collaborations between Japanese universities and genomic research centers are expanding clinical knowledge on JBS-related gene mutations. Moreover, Japan’s emphasis on data-driven healthcare and integration of AI-based genetic analysis tools is accelerating diagnostic efficiency and improving patient outcomes.
India Johanson Blizzard Syndrome Market Insight
The India Johanson Blizzard Syndrome market accounted for the largest market revenue share within Asia-Pacific in 2024, driven by expanding genetic testing capabilities, increasing healthcare investments, and government initiatives supporting rare disease awareness. India’s large population base and improving access to diagnostic technologies are boosting early screening and patient identification. The launch of the National Policy for Rare Diseases (NPRD) is providing structured funding and support for genetic research and patient care. In addition, the emergence of domestic diagnostic laboratories and academic collaborations is enhancing accessibility to affordable testing, making India a rapidly advancing market for Johanson Blizzard Syndrome diagnostics and research.
Johanson Blizzard Syndrome Market Share
The Johanson Blizzard Syndrome industry is primarily led by well-established companies, including:
- Blueprint Genetics Oy. (Finland)
- Fulgent Genetics (U.S.)
- Variantyx, Inc. (U.S.)
- Baylor Genetics (U.S.)
- Labcorp (U.S.)
- Quest Diagnostics (U.S.)
- Invitae Corporation (U.S.)
- GeneDx (U.S.)
- CENTOGENE GmbH (Germany)
- Eurofins Genomics (France)
- DNA Labs India (India)
- CGC Genetics (Portugal)
- Sema4 (U.S.)
- Myriad Genetics (U.S.)
- PerkinElmer (U.S.)
- ARUP Laboratories (U.S.)
- BGI Genomics (China)
- Illumina, Inc. (U.S.)
- LabDNA (U.K.)
What are the Recent Developments in Global Johanson Blizzard Syndrome Market?
- In February 2025, researchers in the Czech Republic published the first molecular confirmation of JBS in that country, identifying two de-novo UBR1 variants in trans this academic collaboration both broadened genotype knowledge and underscored the international research network studying JBS
- In January 2024, a multi-patient case series from Saudi Arabia reported novel UBR1 mutations in four pediatric JBS patients, a publication that reflects cross-institutional collaboration and expands the known UBR1 mutation spectrum information that diagnostic labs and gene-panel providers can use to refine testing and interpretation
- In March 2023, the Else Kröner-Fresenius-Stiftung announced funding for a German research project to model Johanson-Blizzard syndrome in pancreatic organoids (a collaboration between academic labs), aimed at elucidating UBR1-related pancreatic pathology and creating preclinical models for therapy research
- In September 2022, Genomics England updated its PanelApp to include UBR1 across relevant diagnostic panels, improving the integration of JBS testing into routine clinical exome/panel workflows in the U.K. genomic medicine network
- In June 2021, Blueprint Genetics listed and offered a clinical UBR1 single-gene test (definitive molecular diagnostic for JBS) as part of its single-gene test catalogue, expanding clinical access to targeted UBR1 testing for suspected JBS patients
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.