Global Lactate Dehydrogenase Test Market
Market Size in USD Billion
CAGR :
% 
 USD
4.20 Billion
USD
7.84 Billion
2025
2033
USD
4.20 Billion
USD
7.84 Billion
2025
2033
| 2026 –2033 | |
| USD 4.20 Billion | |
| USD 7.84 Billion | |
|
|
|
|
Lactate dehydrogenase Test Market Size
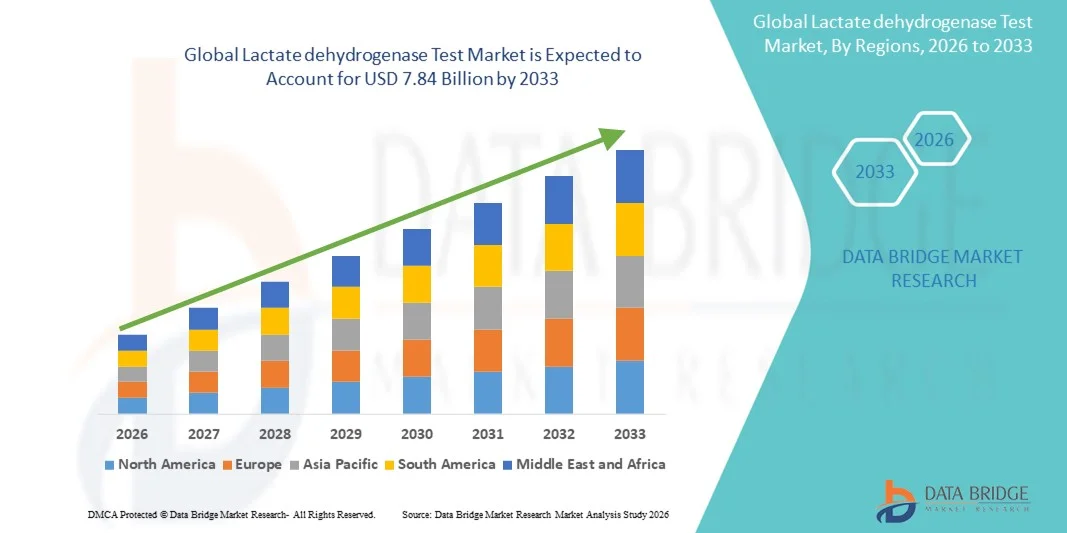
- The global lactate dehydrogenase test market size was valued at USD 4.20 billion in 2025 and is expected to reach USD 7.84 billion by 2033, at a CAGR of 8.10% during the forecast period
- The market growth is largely driven by the increasing prevalence of chronic diseases, cardiovascular disorders, and cancer, along with the rising adoption of point-of-care diagnostics and advanced laboratory testing technologies
- Furthermore, growing awareness among healthcare providers and patients about early disease detection and monitoring, combined with the demand for accurate and rapid biochemical analysis, is positioning LDH tests as a critical diagnostic tool. These converging factors are accelerating the adoption of LDH testing, thereby significantly propelling the market’s growth
Lactate dehydrogenase Test Market Analysis
- Lactate dehydrogenase, providing quantitative measurement of LDH enzyme levels in blood and other body fluids, is increasingly an essential component of modern diagnostic and clinical monitoring protocols in both hospital and laboratory settings due to its rapid results, reliability, and compatibility with automated analyzers
- The escalating demand for lactate dehydrogenase is primarily driven by the rising prevalence of chronic diseases, cardiovascular disorders, liver dysfunctions, and certain cancers, along with the growing adoption of routine biochemical screening and point-of-care diagnostics
- North America dominated the lactate dehydrogenase market with the largest revenue share of 38.9% in 2025, characterized by advanced healthcare infrastructure, high healthcare spending, and strong presence of key diagnostic companies, with the U.S. witnessing significant adoption in hospitals, diagnostic labs, and research centers, driven by innovations in automated and high-throughput testing technologies
- Asia-Pacific is expected to be the fastest growing region in the lactate dehydrogenase market during the forecast period due to increasing healthcare access, rising prevalence of chronic diseases, and expanding laboratory infrastructure
- Instruments segment dominated the lactate dehydrogenase market with a market share of 46.8% in 2025, driven by their high accuracy, reliability, and essential role in conducting both routine and specialized LDH assays across clinical and research laboratories
Report Scope and Lactate dehydrogenase Test Market Segmentation
|
Attributes |
Lactate dehydrogenase Test Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Lactate dehydrogenase Test Market Trends
Advancement Through Automation and High-Throughput Assays
- A significant and accelerating trend in the global lactate dehydrogenase test market is the increasing integration of automated analyzers and high-throughput assay platforms, enhancing speed, accuracy, and laboratory workflow efficiency
- For instance, automated LDH analyzers such as the Roche Cobas series streamline testing by performing multiple assays simultaneously with minimal manual intervention, reducing human error and improving reproducibility
- High-throughput LDH testing enables laboratories to handle larger sample volumes, supporting rapid diagnostics in hospitals, clinical labs, and research facilities. For instance, Beckman Coulter’s high-capacity analyzers allow simultaneous processing of hundreds of samples, accelerating result turnaround times
- The integration of automation with LDH testing systems also supports remote data access and digital reporting, allowing healthcare professionals to monitor patient results in real time and make timely clinical decisions
- This trend toward more automated, accurate, and scalable LDH testing solutions is fundamentally reshaping laboratory operations and diagnostic workflows. For instance, laboratories adopting automated LDH analyzers are experiencing improved productivity, reduced manual labor, and faster reporting of critical results
- The demand for automated and high-throughput LDH testing platforms is growing rapidly across hospitals, diagnostic labs, and research centers, as institutions prioritize efficiency, accuracy, and timely clinical decision-making
- Integration with laboratory information management systems (LIMS) is becoming increasingly common, allowing seamless tracking, analysis, and storage of LDH test data for enhanced patient management and research capabilities. For instance, hospitals using LIMS-enabled LDH assays can generate trend reports and alert clinicians to abnormal results automatically
- There is a rising trend toward multiplexing LDH tests with other enzyme markers in a single assay panel, providing comprehensive diagnostic insights while saving time and reducing sample volumes. For instance, some advanced biochemical panels now combine LDH, AST, and ALT measurements for liver function assessment
Lactate dehydrogenase Test Market Dynamics
Driver
Rising Demand for Early Disease Detection and Monitoring
- The increasing prevalence of chronic diseases, liver disorders, cardiovascular conditions, and cancers, coupled with the growing importance of early diagnosis and routine monitoring, is a significant driver for the heightened demand for lactate dehydrogenase tests
- For instance, in March 2025, Abbott Laboratories launched enhanced LDH testing kits integrated with their Alinity automated system, supporting faster and more reliable disease monitoring in clinical laboratories
- As clinicians emphasize early detection and ongoing patient monitoring, LDH tests offer rapid and accurate quantification of enzyme levels, helping to identify disease progression or therapeutic response
- Furthermore, the growing adoption of point-of-care testing and integration with hospital information systems is making LDH tests an integral component of diagnostic protocols
- The convenience of automated testing, faster result availability, and ability to track patient biomarkers over time are key factors propelling the adoption of LDH tests in clinical and research settings. The trend toward data-driven healthcare and personalized monitoring further supports market growth
- Increasing research activities in oncology, cardiology, and liver disease areas are driving demand for LDH testing as a reliable biomarker for disease prognosis and therapeutic monitoring. For instance, clinical studies now frequently incorporate LDH measurements to evaluate treatment efficacy and disease progression
- Growing collaborations between diagnostic companies and hospitals or research institutions are accelerating the development of novel LDH assays and testing platforms, further expanding market opportunities. For instance, partnerships between Siemens Healthineers and leading medical centers focus on advancing LDH assay automation and throughput
Restraint/Challenge
Sample Variability and Regulatory Compliance Hurdle
- Concerns surrounding sample variability, pre-analytical errors, and differences in assay methods pose a significant challenge to the broader adoption of lactate dehydrogenase tests. LDH levels can be affected by hemolysis, storage conditions, and patient-specific factors, impacting test accuracy
- For instance, high variability in sample handling has led some laboratories to adopt stricter quality control measures, increasing operational complexity and cost
- Addressing these pre-analytical and analytical challenges through standardized protocols, validated assay kits, and operator training is crucial for ensuring reliable test results. For instance, manufacturers such as Roche and Beckman Coulter provide detailed guidelines and QC materials to reduce variability
- In addition, navigating stringent regulatory requirements for in-vitro diagnostics, including approvals from FDA, CE, and other authorities, can delay product launches and increase compliance costs
- While new LDH assays are continually developed to improve accuracy and throughput, the combination of sample variability, regulatory hurdles, and high costs for advanced instruments can hinder adoption, particularly in resource-limited regions. Overcoming these challenges through quality standardization, regulatory support, and cost-effective solutions will be vital for sustained market growth
- Limited awareness and technical expertise in smaller diagnostic centers, particularly in emerging regions, can restrict the adoption of advanced LDH testing technologies. For instance, some regional laboratories rely on manual or semi-automated methods due to lack of training or infrastructure
- Potential interference from other enzymes or metabolites in patient samples may lead to inaccurate LDH readings, creating a need for more robust assay designs and validation studies. For instance, hemolyzed samples or elevated bilirubin levels can distort LDH results, necessitating careful sample handling and assay selection
Lactate dehydrogenase Test Market Scope
The market is segmented on the basis of product, type, indications, and assay type.
- By Product
On the basis of product, the lactate dehydrogenase (LDH) test market is segmented into instruments, consumables, and others. Instruments segment dominated the market with the largest revenue share of 46.8% in 2025, driven by the essential role of analyzers and automated systems in conducting LDH tests with high accuracy and reproducibility. Clinical laboratories and hospitals prioritize instrument-based LDH testing for rapid turnaround times, large sample volumes, and integration with laboratory information systems. Instruments also provide flexibility for running multiple assay types simultaneously, which is critical in multi-test panels used for disease monitoring. Major players such as Roche, Abbott, and Beckman Coulter continue to innovate in automated analyzers, enhancing workflow efficiency and minimizing manual errors. The high reliability and standardization offered by instrument-based LDH tests make them the preferred choice for clinical diagnostics. In addition, instruments facilitate advanced reporting, real-time data tracking, and connectivity with hospital networks, further consolidating their dominance.
Consumables segment is expected to witness the fastest growth from 2026 to 2033, fueled by increasing adoption in point-of-care testing and expanding use in decentralized laboratories. Consumables include reagents, assay kits, and sample preparation materials necessary for LDH analysis. Their growth is supported by rising demand for quick, cost-effective, and disposable solutions, especially in emerging regions where full-scale instruments may be limited. In addition, continuous innovation in pre-prepared assay kits and single-use reagents enhances test accuracy and ease of use, attracting smaller clinics and research facilities. The growing trend of combining LDH consumables with other enzyme assays for multi-marker diagnostics further drives market expansion.
- By Type
On the basis of type, the market is segmented into LD1, LD2, LD3, LD4, and LD5 isoenzymes. LD1 segment dominated the market in 2025 due to its critical role in diagnosing cardiac conditions and hemolytic disorders. LD1 levels are closely monitored in patients with myocardial infarction, making it a routine biomarker in cardiology labs. Laboratories often prefer LD1 testing for its well-established clinical relevance and the availability of standardized assays. The widespread clinical adoption of LD1 testing in hospitals, research centers, and diagnostic labs ensures consistent demand. Automated instruments and dedicated kits designed for LD1 detection further improve accuracy, speed, and reproducibility, reinforcing its market dominance. Moreover, LD1 assays are frequently included in multi-enzyme panels, increasing their usage in routine screenings.
LD5 segment is anticipated to witness the fastest growth during the forecast period due to its importance in liver disease diagnosis and monitoring. LD5 levels are indicative of hepatocellular damage, making it highly relevant in oncology and liver disorder testing. Rising prevalence of liver diseases, coupled with increasing awareness of early detection, is boosting demand for LD5-specific assays. Advanced reagents and assay kits targeting LD5 are becoming more accessible in emerging markets, further driving adoption. In addition, research applications exploring LD5 as a biomarker for liver injury and drug toxicity studies are fueling market growth.
- By Indications
On the basis of indications, the market is segmented into cerebrovascular diseases, liver diseases, blood disorders, and others. Blood Disorders segment dominated the market in 2025 due to the extensive use of LDH testing in diagnosing hemolytic anemia, leukemia, and other blood-related disorders. LDH levels serve as an important biomarker for assessing cell damage and disease progression in hematological conditions. Routine monitoring in hospitals and diagnostic labs ensures steady demand. Laboratory automation and standardized protocols improve the accuracy and reproducibility of LDH tests for blood disorders, consolidating its position. The segment also benefits from the growing adoption of multi-parameter panels where LDH testing is combined with other hematology markers. In addition, academic and clinical research into new blood disorder treatments continuously relies on LDH assays, supporting market dominance.
Liver Diseases segment is expected to witness the fastest growth from 2026 to 2033 due to increasing incidence of liver cirrhosis, hepatitis, and non-alcoholic fatty liver disease. LDH testing is crucial for evaluating hepatocellular injury and monitoring disease progression. Growing awareness among healthcare professionals and patients regarding early detection and treatment of liver conditions is fueling demand. Emerging markets are witnessing increased adoption of LDH assays for liver indications due to expanding hospital infrastructure and diagnostic capabilities. Innovations in high-throughput assays and point-of-care testing further accelerate adoption. In addition, research on liver-targeted therapies and drug-induced liver injury monitoring is supporting segment growth.
- By Assay Type
On the basis of assay type, the market is segmented into LDH Cytotoxicity Colorimetric Assay, LDH Cytotoxicity Fluorometric Assay, SRB Assay, and WST Assay. LDH Cytotoxicity Colorimetric Assay segment dominated the market in 2025, driven by its simplicity, cost-effectiveness, and compatibility with standard laboratory equipment. It is widely used in clinical diagnostics, cytotoxicity testing, and drug development studies due to reliable quantitative results. Laboratories favor colorimetric assays for routine LDH analysis, as they offer consistent performance and are easy to interpret. The availability of standardized kits and reagents enhances reproducibility and reduces operator dependency. Major diagnostic companies continue to innovate in this assay format, improving sensitivity and throughput. Its dominance is reinforced by extensive adoption in research institutions, pharmaceutical labs, and hospital-based testing facilities.
LDH Cytotoxicity Fluorometric Assay segment is expected to witness the fastest growth from 2026 to 2033 due to its higher sensitivity, suitability for low-volume samples, and applicability in high-throughput screening. Fluorometric assays enable detection of minimal LDH activity, making them ideal for advanced research and early disease detection studies. Rising demand from oncology, pharmacology, and biotechnology sectors drives adoption. In addition, fluorometric assays integrate well with automated and multiplexed platforms, enhancing efficiency and scalability. Innovations in fluorogenic substrates and miniaturized assay formats further boost segment growth, particularly in research-intensive regions.
Lactate dehydrogenase Test Market Regional Analysis
- North America dominated the lactate dehydrogenase market with the largest revenue share of 38.9% in 2025, characterized by advanced healthcare infrastructure, high healthcare spending, and strong presence of key diagnostic companies, with the U.S. witnessing significant adoption in hospitals, diagnostic labs, and research centers, driven by innovations in automated and high-throughput testing technologies
- Healthcare providers and diagnostic laboratories in the region prioritize rapid, accurate, and reliable LDH testing for early disease detection, treatment monitoring, and research applications, contributing to steady demand
- This widespread adoption is further supported by strong government healthcare spending, high awareness among clinicians regarding biomarker-based diagnostics, and the growing preference for integrated laboratory information systems, establishing LDH testing as a key tool in patient management across hospitals, research centers, and clinical labs
U.S. Lactate dehydrogenase Test Market Insight
The U.S. lactate dehydrogenase test market captured the largest revenue share of 82% in 2025 within North America, fueled by the rapid adoption of automated laboratory systems and the growing emphasis on early disease detection. Healthcare providers increasingly prioritize LDH testing for monitoring chronic diseases, liver disorders, and cardiovascular conditions. The growing integration of LDH assays with laboratory information management systems (LIMS) and point-of-care devices further propels market growth. In addition, research institutions and hospitals are adopting high-throughput LDH testing platforms to support clinical trials and advanced diagnostics. The widespread availability of standardized assay kits and reagents ensures reliable and reproducible results, reinforcing market dominance. Moreover, increasing collaborations between diagnostic companies and U.S. hospitals are contributing to technological advancements and broader adoption.
Europe Lactate Dehydrogenase Test Market Insight
The Europe lactate dehydrogenase test market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing prevalence of liver diseases, blood disorders, and cardiovascular conditions. Rising awareness among healthcare professionals and patients regarding early diagnosis and monitoring is fostering adoption of LDH testing. European hospitals and diagnostic laboratories are incorporating LDH assays into routine panels for chronic disease management. The presence of well-established healthcare infrastructure and government support for clinical diagnostics further drives growth. In addition, research activities in oncology and hematology across the region are boosting demand for LDH testing. Increasing adoption in both hospital and private laboratory settings, alongside advanced automation, ensures consistent market expansion.
U.K. Lactate Dehydrogenase Test Market Insight
The U.K. lactate dehydrogenase test market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the rising focus on early detection of chronic and cardiovascular diseases. Increasing hospital automation, coupled with a growing demand for accurate and rapid biomarker-based diagnostics, supports the adoption of LDH testing. In addition, clinical research and pharmaceutical trials in the U.K. are contributing to consistent demand for high-quality LDH assays. The robust healthcare system, combined with widespread laboratory infrastructure, enables large-scale testing and efficient integration of LDH results into patient care. Rising awareness among clinicians about disease monitoring and prognosis further encourages utilization. Moreover, the growing use of point-of-care LDH testing in hospitals and private clinics is enhancing the market potential.
Germany Lactate Dehydrogenase Test Market Insight
The Germany lactate dehydrogenase test market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing prevalence of liver and cardiovascular diseases, along with growing research activities in hematology and oncology. German hospitals and diagnostic laboratories emphasize accurate, high-throughput testing, making LDH assays a key diagnostic tool. Integration of LDH testing with automated analyzers and laboratory information systems is becoming increasingly prevalent. Local emphasis on quality, precision, and regulatory compliance promotes adoption of standardized LDH kits and instruments. In addition, advanced clinical research and pharmaceutical studies in Germany drive demand for LDH testing platforms. Hospitals and research centers are increasingly relying on LDH as a routine biomarker for monitoring disease progression and treatment efficacy.
Asia-Pacific Lactate Dehydrogenase Test Market Insight
The Asia-Pacific lactate dehydrogenase test market is poised to grow at the fastest CAGR of 25% during the forecast period of 2026 to 2033, driven by increasing prevalence of chronic and lifestyle-related diseases, rising healthcare awareness, and expanding diagnostic infrastructure in countries such as China, Japan, and India. Growing adoption of automated laboratory systems and point-of-care devices supports faster and more accurate LDH testing. Government initiatives to strengthen healthcare infrastructure and improve diagnostic capabilities are boosting market penetration. The rising number of private and public diagnostic laboratories is increasing accessibility to LDH tests. In addition, ongoing research in oncology and liver disorders across APAC further supports adoption. Expansion of regional manufacturing of LDH assay kits and reagents is reducing costs and enhancing availability, driving market growth.
Japan Lactate Dehydrogenase Test Market Insight
The Japan lactate dehydrogenase test market is gaining momentum due to the country’s advanced healthcare infrastructure, growing geriatric population, and focus on preventive diagnostics. Japanese hospitals and clinical labs increasingly rely on LDH testing for monitoring liver function, cardiovascular health, and oncology-related conditions. Integration of LDH assays with automated systems and digital reporting platforms supports rapid diagnostics and efficient patient management. Rising adoption of high-throughput and point-of-care testing solutions further fuels growth. Moreover, clinical research institutions are incorporating LDH testing in studies for drug development and disease monitoring. Japan’s technological advancements and emphasis on healthcare innovation are expected to continue supporting market expansion.
India Lactate Dehydrogenase Test Market Insight
The India lactate dehydrogenase test market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to increasing prevalence of lifestyle-related diseases, growing healthcare awareness, and expanding hospital and laboratory infrastructure. India is witnessing a rising number of diagnostic centers adopting automated LDH testing systems for faster and accurate results. The push toward smart healthcare facilities and increasing use of point-of-care devices are key factors propelling market growth. Affordable LDH assay kits and instruments, alongside domestic manufacturing capabilities, enhance accessibility across urban and semi-urban regions. Furthermore, growing research initiatives in liver disease, oncology, and cardiovascular health are driving demand for LDH tests. Rising investment in healthcare technology and collaborations between diagnostic firms and hospitals continue to support market expansion.
Lactate dehydrogenase Test Market Share
The Lactate dehydrogenase Test industry is primarily led by well-established companies, including:
- Abbott (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- F. Hoffmann La Roche Ltd. (Switzerland)
- Sysmex Corporation (Japan)
- BIOMÉRIEUX (France)
- Siemens Healthineers AG (Germany)
- Danaher (U.S.)
- Randox Laboratories Ltd. (U.K.)
- QIAGEN (Netherlands)
- Eurofins Scientific SE (Luxembourg)
- Quest Diagnostics Incorporated (U.S.)
- PerkinElmer (U.S.)
- Abcam plc (U.K.)
- Merck KGaA (Germany)
- Abnova Corporation (Taiwan)
- Accurex Biomedical Pvt. Ltd. (India)
- LifeSpan BioSciences Inc. (U.S.)
- Sekisui Diagnostics LLC (U.S./Japan)
- Bio Rad Laboratories, Inc. (U.S.)
- DiaSorin S.p.A. (Italy)
What are the Recent Developments in Global Lactate dehydrogenase Test Market?
- In July 2025, Siemens Healthineers became the first in vitro diagnostics (IVD) manufacturer to earn the My Green Lab ACT Ecolabel for over 150 clinical chemistry reagents and analyzers, including those used in in‑vitro diagnostic testing (which encompasses LDH and other clinical assays), highlighting environmental responsibility and performance of reagents and potentially boosting adoption in clinical lab
- In May 2025, a study published in Scientific Reports demonstrated that serum lactate dehydrogenase levels serve as a strong prognostic marker for 90‑day mortality among connective tissue disease patients receiving glucocorticoids and hospitalized with pneumonia, reinforcing the clinical relevance of LDH testing in risk stratification and patient management in complex disease settings
- In December 2023, Medtronic, a leading medical device company, launched a rapid lactate dehydrogenase (LDH) test designed to deliver faster results for LDH levels, enabling quicker diagnostic decision‑making in critical care settings and aiming to improve patient outcomes and reduce costs associated with delayed diagnosis
- In August 2023, Siemens Healthineers entered into a strategic collaboration with Abcam, a life science tools and reagents company, to develop advanced LDH assays for Siemens’ diagnostic platforms, aiming to improve precision, efficiency, and diagnostic accuracy of LDH testing
- In August 2021, DiaSys Diagnostic Systems GmbH announced the commercial launch of LDH 21 FS, a new reagent for the determination of lactate dehydrogenase (LDH) that offers improved calibration, onboard stability, a wider measuring range, high precision at clinical cut‑offs, and reduced interference by common blood components; the reagent is compatible with a wide range of automated clinical chemistry analyzers and supports more reliable LDH measurement across laboratories.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.