Global Lal Testing Market
Market Size in USD Million
CAGR :
% 
 USD
229.94 Million
USD
458.18 Million
2024
2032
USD
229.94 Million
USD
458.18 Million
2024
2032
| 2025 –2032 | |
| USD 229.94 Million | |
| USD 458.18 Million | |
|
|
|
|
What is the Global LAL Testing Market Size and Growth Rate?
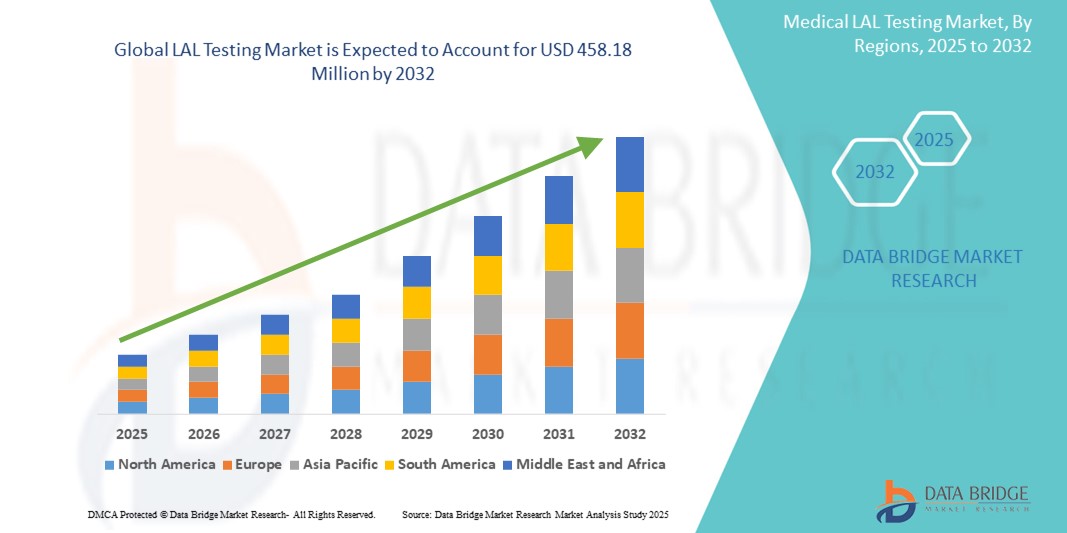
- The global LAL testing market size was valued at USD 229.94 million in 2024 and is expected to reach USD 458.18 million by 2032, at a CAGR of 9.00% during the forecast period
- The global LAL testing market is expected to witness significant growth in the coming years, driven by factors such as the increasing prevalence of infectious diseases, growing concerns regarding the safety of pharmaceutical and medical device products, and the rising adoption of LAL testing in various industries
What are the Major Takeaways of LAL Testing Market?
- The pharmaceutical industry is a major contributor to market growth, as LAL testing is a crucial step in ensuring the safety and quality of drugs. In addition, the medical device industry is increasingly adopting LAL testing to meet regulatory requirements and enhance product safety
- North America dominated the LAL testing market with the largest revenue share of 42.8% in 2024, driven by stringent regulatory requirements for endotoxin testing across pharmaceutical and medical device industries
- Asia-Pacific LAL Testing market is projected to grow at the fastest CAGR of 12.6% from 2025 to 2032, driven by the rapid expansion of pharmaceutical manufacturing, particularly in countries such as China, India, and Japan
- The Gel Clot Endotoxin Test segment dominated the market with the largest market revenue share of 48.6% in 2024, owing to its widespread regulatory acceptance, cost-effectiveness, and simplicity in qualitative endotoxin detection
Report Scope and LAL Testing Market Segmentation
|
Attributes |
LAL Testing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
What is the Key Trend in the LAL Testing Market?
“Biotechnology Advancements and Shift toward Recombinant LAL Testing”
- A significant and evolving trend in the global LAL testing market is the growing shift towards recombinant LAL alternatives and biotechnological innovations, driven by sustainability concerns and regulatory support. The rising demand for endotoxin testing, especially in pharmaceutical and medical device industries, is accelerating the adoption of synthetic and animal-free LAL testing solutions
- For instance, Lonza (Switzerland) has introduced PyroGene recombinant Factor C (rFC) assay, providing a sustainable and reliable alternative to traditional LAL testing, reducing dependence on horseshoe crab blood while ensuring regulatory compliance in quality control
- Increasing regulatory support from organizations such as the U.S. FDA and European Pharmacopoeia, promoting alternative endotoxin detection methods, is pushing pharmaceutical manufacturers to adopt advanced testing technologies that align with both efficacy and ethical standards
- Recombinant LAL testing methods alleviate ecological concerns and enhance accuracy, batch consistency, and reduce supply chain risks associated with horseshoe crab harvesting. The market is witnessing broader application of such technologies in biologics, vaccines, and injectable drug production
- This trend is transforming the endotoxin detection landscape, with companies such as Charles River Laboratories (U.K.) and Thermo Fisher Scientific (U.S.) investing in scalable, next-generation LAL testing platforms that support stringent quality control demands across pharma and biotech sectors
- As the industry embraces innovation, demand for recombinant, reliable, and environmentally responsible LAL testing solutions is expected to surge, particularly in regions with strong pharmaceutical manufacturing such as North America, Europe, and Asia-Pacific
What are the Key Drivers of LAL Testing Market?
- The increasing prevalence of injectable drugs, vaccines, and biopharmaceutical products is a primary driver for the growing demand for LAL testing, given its critical role in ensuring product sterility and patient safety
- For instance, in February 2024, WuXi AppTec (China) expanded its endotoxin testing capabilities to support the surging global demand for sterile biologics, enhancing the company’s comprehensive quality control service portfolio. Such developments are driving industry growth
- Stringent regulatory frameworks from bodies such as the U.S. FDA, EMA, and Pharmacopeial organizations mandate routine endotoxin testing for pharmaceuticals, medical devices, and biological products, ensuring consistent market demand
- The rise in global healthcare spending, increasing pharmaceutical R&D, and the ongoing demand for high-purity injectable therapies, including monoclonal antibodies, cell and gene therapies, and COVID-19 vaccines, further propel the need for reliable LAL testing solutions
- Furthermore, technological advancements leading to faster, more sensitive, and automated LAL assays enhance operational efficiency and reduce testing turnaround time, encouraging widespread adoption across quality control laboratories
Which Factor is challenging the Growth of the LAL Testing Market?
- The over-reliance on horseshoe crab blood for traditional LAL testing remains a major ecological and ethical concern, leading to supply constraints and regulatory scrutiny that may affect market stability
- For instance, concerns over horseshoe crab populations along the U.S. Atlantic coast have sparked debates among conservation groups, regulators, and industry stakeholders, emphasizing the need for alternative testing solutions
- Moreover, despite technological progress, limited global awareness and slower regulatory adoption of recombinant LAL alternatives in some markets continue to restrict widespread use. Regulatory harmonization across regions is still evolving, creating fragmented approval pathways
- High initial investment costs for advanced endotoxin detection platforms and the need for technical expertise can also act as barriers, particularly for small to mid-sized pharmaceutical manufacturers in emerging markets
- To overcome these challenges, companies must focus on increasing awareness of sustainable testing options, collaborate with regulatory bodies to accelerate global harmonization, and invest in cost-effective, scalable solutions to broaden market accessibility
How is the LAL Testing Market Segmented?
The market is segmented on the basis of testing methods and application.
- By Testing Methods
On the basis of testing methods, the LAL testing market is segmented into Gel Clot Endotoxin Test, Chromogenic Endotoxin Test, and Turbidimetric Endotoxin Test. The Gel Clot Endotoxin Test segment dominated the market with the largest market revenue share of 48.6% in 2024, owing to its widespread regulatory acceptance, cost-effectiveness, and simplicity in qualitative endotoxin detection. Pharmaceutical manufacturers and medical device companies often rely on Gel Clot tests due to their proven track record, ease of use, and suitability for routine lot-release testing in sterile production environments. The method’s minimal instrumentation requirements make it especially popular among small to mid-sized manufacturers and for initial product screenings.
The Chromogenic Endotoxin Test segment is expected to witness the fastest CAGR from 2025 to 2032, fueled by increasing demand for quantitative, highly sensitive endotoxin detection in biologics, vaccines, and injectable pharmaceuticals. Chromogenic tests offer precise, real-time endotoxin measurement, supporting stricter quality control standards in high-purity manufacturing environments. The growing complexity of advanced therapies is also propelling the adoption of this method across pharma and biotechnology industries.
- By Application
On the basis of application, the LAL testing market is segmented into Medical Device Manufacturing and Pharmaceutical Manufacturing. The Pharmaceutical Manufacturing segment accounted for the largest market revenue share of 65.4% in 2024, driven by strict global regulatory requirements for endotoxin testing in injectable drugs, biologics, vaccines, and parenteral therapies. The rising production of biologics, monoclonal antibodies, and cell & gene therapies necessitates reliable endotoxin control, making LAL testing an indispensable part of pharmaceutical quality assurance.
The Medical Device Manufacturing segment is anticipated to register the fastest growth rate from 2025 to 2032, as increasing regulatory scrutiny mandates endotoxin testing for implantable devices, surgical instruments, and other critical healthcare products. The heightened demand for sterile, high-quality medical devices, particularly in orthopedics, cardiology, and neurology, is driving the adoption of LAL testing in this sector. The surge in global healthcare infrastructure expansion further accelerates market growth across device manufacturing applications.
Which Region Holds the Largest Share of the LAL Testing Market?
- North America dominated the LAL testing market with the largest revenue share of 42.8% in 2024, driven by stringent regulatory requirements for endotoxin testing across pharmaceutical and medical device industries
- The region benefits from a well-established biopharmaceutical sector, heightened emphasis on patient safety, and significant investments in quality control for injectable drugs and implantable medical devices
- The demand for LAL testing is further supported by a growing pipeline of biologics, rising clinical trial activities, and strict U.S. FDA mandates for endotoxin testing, making North America the global leader in this space
U.S. LAL Testing Market Insight
U.S. LAL testing market captured the largest revenue share of 78% within North America in 2024, driven by the country’s dominant pharmaceutical manufacturing sector and world-leading biotech innovation hubs. The increased production of biologics, vaccines, and sterile injectable drugs has amplified demand for accurate endotoxin detection methods. Moreover, stringent regulatory frameworks from the U.S. FDA and the USP for pyrogen and endotoxin testing continue to fuel market growth. The nation’s robust medical device industry and a surge in surgical procedures also contribute to LAL Testing adoption.
Europe LAL Testing Market Insight
Europe LAL testing market is expected to expand at a substantial CAGR during the forecast period, supported by strong regulatory oversight from entities such as the European Medicines Agency (EMA) and the rise of advanced therapy medicinal products (ATMPs). Growing biologics development, combined with the region’s focus on pharmaceutical quality control, is accelerating LAL Testing demand. Furthermore, initiatives aimed at reducing contamination risks in healthcare settings and a strong medical device manufacturing base are fostering market expansion across both established and emerging European markets.
U.K. LAL Testing Market Insight
U.K. LAL testing market is projected to grow at a notable CAGR, supported by the country’s active pharmaceutical R&D ecosystem, advanced biomanufacturing capabilities, and regulatory alignment with international standards for endotoxin control. The growing production of biologics, vaccines, and cell-based therapies is increasing the demand for LAL Testing. Furthermore, a rising emphasis on healthcare safety and contamination prevention across hospitals and laboratories further boosts the market.
Germany LAL Testing Market Insight
The Germany LAL testing market is poised for steady growth, driven by its status as one of Europe’s leading hubs for pharmaceutical production, medical device innovation, and biotechnology research. Heightened demand for sterile injectable drugs and implantable devices, coupled with Germany’s strong regulatory compliance culture, is fueling LAL Testing adoption. The nation’s push towards high-tech healthcare solutions and a growing focus on biologics manufacturing also contribute to market expansion.
Which Region is the Fastest Growing Region in the LAL Testing Market?
Asia-Pacific LAL testing market is projected to grow at the fastest CAGR of 12.6% from 2025 to 2032, driven by the rapid expansion of pharmaceutical manufacturing, particularly in countries such as China, India, and Japan. The region’s increasing investments in biotech, rising exports of injectable drugs and medical devices, and evolving regulatory standards for endotoxin testing are key growth factors. APAC’s expanding healthcare infrastructure and focus on domestic vaccine production also fuel LAL Testing demand, especially post-pandemic.
Japan LAL Testing Market Insight
The Japan LAL testing market is gaining momentum, fueled by the country's robust pharmaceutical manufacturing, high healthcare quality standards, and focus on advanced therapies. The growing demand for sterile injectable products, combined with government support for biologics and regenerative medicine, is expanding LAL Testing applications. Japan's emphasis on patient safety, coupled with innovations in contamination control, positions the market for steady growth.
China LAL Testing Market Insight
China LAL testing market accounted for the largest revenue share in Asia-Pacific in 2024, supported by the nation’s booming pharmaceutical production, growing biotech sector, and rising exports of injectable medicines and medical devices. Stringent government regulations for product safety, the push towards self-reliance in vaccine and biologics manufacturing, and strong investments in healthcare R&D continue to propel the demand for reliable endotoxin testing methods such as LAL Testing across China.
Which are the Top Companies in LAL Testing Market?
The LAL testing industry is primarily led by well-established companies, including:
- Pacific BioLabs (U.S.)
- Lonza (Switzerland)
- Nelson Laboratories, LLC (U.S.)
- Bio-Synthesis Inc (U.S.)
- Biogenuix (India)
- GenScript (U.S.)
- Thermo Fisher Scientific, Inc. (U.S.)
- SGS Société Générale de Surveillance SA (Switzerland)
- WuXi AppTec (China)
- Sartorius AG (Germany)
- AstraZeneca (U.K.)
- Novasep (France)
- Merck KGaA (Germany)
- Charles River Laboratories (U.K.)
What are the Recent Developments in Global LAL Testing Market?
- In December 2024, Ellab completed the acquisition of PharmaProcess in Italy and Switzerland, aimed at strengthening its life science service portfolio. The integration combines PharmaProcess' regulatory knowledge with Ellab’s compliance solutions, enabling comprehensive support for pharmaceutical and biotechnology companies across both countries. This move is expected to expand Ellab’s regional footprint and enhance service capabilities for endotoxin and quality testing requirements
- In September 2024, Lonza Walkersville initiated the expansion of its endotoxin assay production site in Walkersville, Maryland, with an 18,000-square-foot facility upgrade. This expansion is designed to increase production capacity in response to the growing global demand for endotoxin assays essential for ensuring the safety of injectable drugs and medical devices. The development will further strengthen Lonza’s position in the LAL Testing market
- In June 2024, FUJIFILM Wako Pure Chemicals unveiled two advanced endotoxin and pyrogen detection solutions: the LumiMAT Pyrogen Detection Kit, a next-generation monocyte activation test, and PYROSTAR Neo+, a recombinant protein reagent for bacterial endotoxin testing, which became globally available in July 2024. These innovations enhance the accuracy and efficiency of endotoxin detection, supporting safety standards across pharmaceutical and medical applications
- In March 2024, AmeboGenesis achieved a significant breakthrough in sustainable Amebocyte production by introducing a pioneering technology that enables the laboratory production of bio-identical Amebocytes for LAL testing, eliminating the need to harvest horseshoe crab blood. As horseshoe crab blood is critical for Limulus Amebocyte Lysate (LAL) production, this advancement promotes sustainability while ensuring the safety of injectable drugs, vaccines, and medical devices worldwide
- In October 2023, Lonza launched two Monocyte Activation Test (MAT) systems, the PyroCell MAT Human Serum (HS) Rapid System and the PyroCell MAT Rapid System, designed to deliver faster, reliable pyrogen testing for pharmaceutical manufacturers. These systems enhance efficiency in detecting pyrogens, contributing to improved safety and regulatory compliance in drug and medical device production processes
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.