Global Lysosomal Storage Disorder Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
10.76 Billion
USD
21.93 Billion
2024
2032
USD
10.76 Billion
USD
21.93 Billion
2024
2032
| 2025 –2032 | |
| USD 10.76 Billion | |
| USD 21.93 Billion | |
|
|
|
|
Lysosomal Storage Disorder Drugs Market Analysis
The global lysosomal storage disorder drugs market encompasses the commercial sector for pharmaceuticals developed to diagnose, treat, and manage various lysosomal storage disorders, which are rare genetic conditions typically characterized by the accumulation of undigested substances within lysosomes due to enzyme deficiencies. This market includes a range of therapeutic products, such as enzyme replacement therapies, substrate reduction therapies, and gene therapies, aimed at addressing the diverse clinical manifestations of LSDs, such as Gaucher disease, Fabry disease, and Pompe disease. With increasing awareness, advancements in research and technology, and a growing number of pipeline therapies, this market is poised for significant expansion. In addition, rising healthcare expenditure and initiatives to improve access to rare disease treatments further drive market growth, presenting both opportunities and challenges for pharmaceutical companies and healthcare providers.
Lysosomal Storage Disorder Drugs Market Size
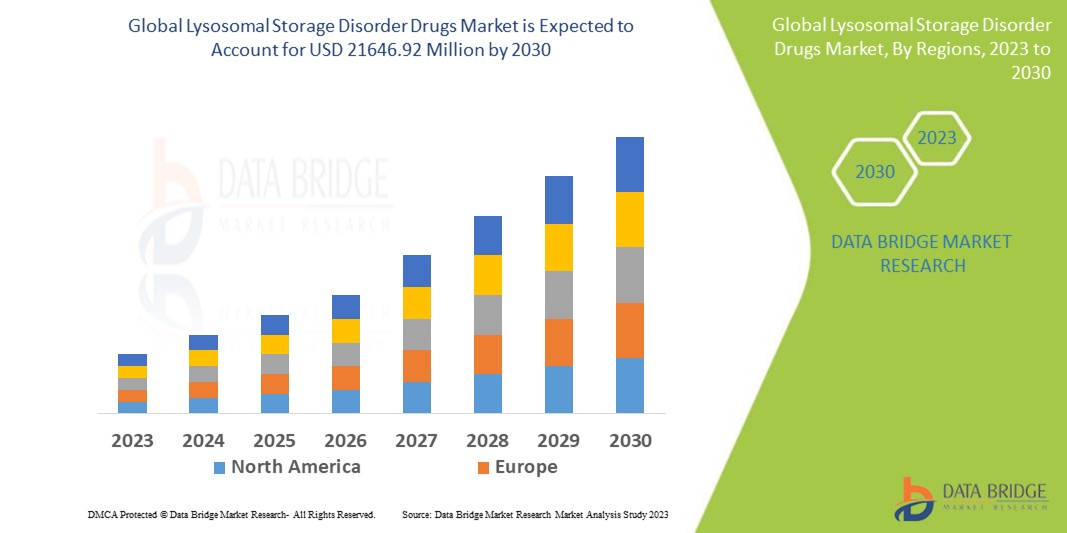
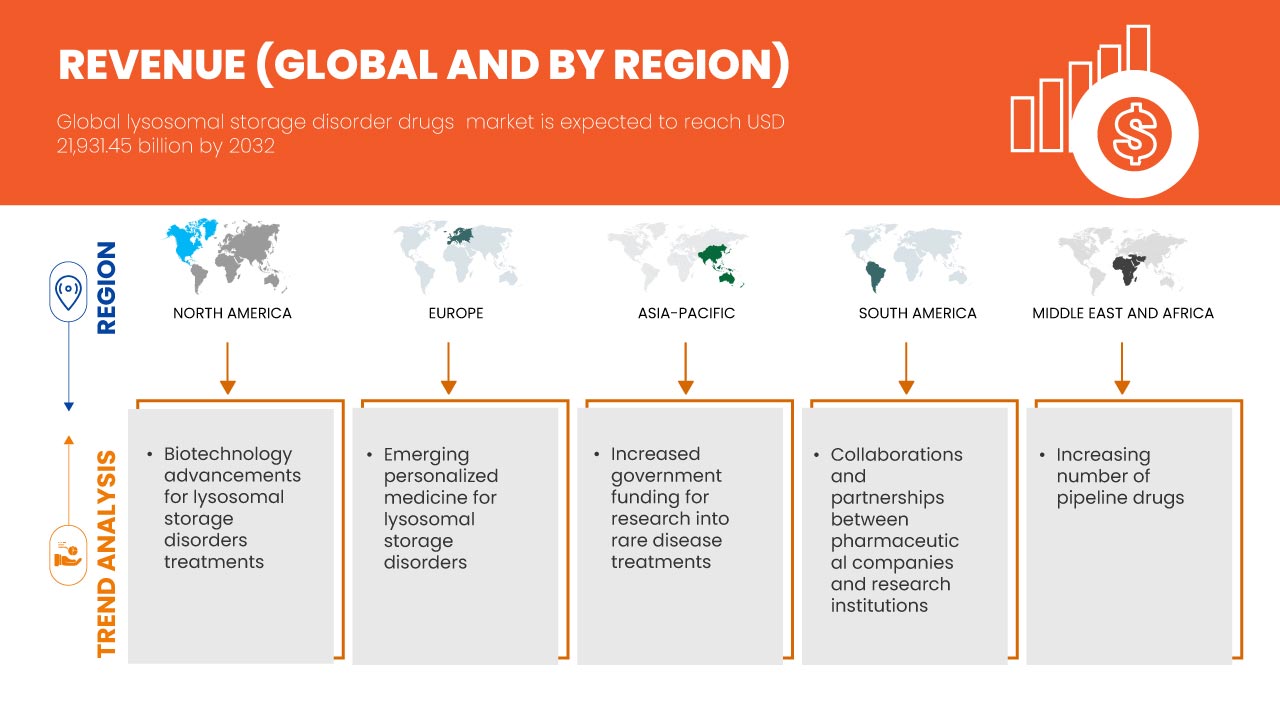
Global lysosomal storage disorder drugs market size was valued at USD 10.76 billion in 2024 and is projected to reach USD 21.93 billion by 2032, growing with a CAGR of 9.4% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework.
Lysosomal Storage Disorder Drugs Market Trends
“Biotechnology Advancements for Lysosomal Storage Disorders Treatments”
Advancements in biotechnology have played a pivotal role in transforming the treatment landscape for Lysosomal Storage Disorders (LSDs), providing patients with more effective and tailored treatment options. Enzyme Replacement Therapy (ERT) has revolutionized the treatment of Lysosomal Storage Disorders (LSDs) by directly addressing the enzyme deficiencies that cause these conditions. In LSD patients, the lack of specific enzymes leads to the accumulation of toxic substances in cells, damaging organs and tissues. ERT works by administering synthetic enzymes to compensate for the missing ones, improving metabolic functions and alleviating symptoms. Over the years, ERT has advanced significantly, with new, more effective formulations and delivery methods that improve enzyme absorption and reduce side effects. These innovations have resulted in better clinical outcomes, this trend slowed disease progression, reduced organ damage, and enhanced patient quality of life.
Report Scope and Lysosomal Storage Disorder Drugs Market Segmentation
|
Attributes |
Lysosomal Storage Disorder Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, U.K., Italy, France, Spain, Russia, Switzerland, Turkey, Belgium, Netherlands, Denmark, Sweden, Poland, Norway, Finland, rest of Europe, Japan, China, South Korea, India, Singapore, Thailand, Indonesia, Malaysia, Philippines, Australia, New Zealand, Vietnam, Taiwan, rest of Asia-Pacific, Brazil, Argentina, rest of South America, South Africa, Egypt, Bahrain, United Arab Emirates, Kuwait, Oman, Qatar, Saudi Arabia, and rest of Middle East and Africa |
|
Key Market Players |
Sanofi (France), BioMarin (U.S.), Pfizer Inc. (U.S.), Amicus Therapeutics, Inc. (U.S.), Takeda Pharmaceutical Company Limited (Japan), Ultragenyx Pharmaceutical Inc. (U.S.), Orchard Therapeutics plc (U.K.), Spur Therapeutics (U.K.), Sangamo Therapeutics (U.S.), Protalix BioTherapeutics Inc. (Israel), CHIESI Farmaceutici S.p.A. (Italy), Forge Biologics (U.S.), Denali Therapeutics (U.S.), REGENXBIO INC. (U.S.), and JCR Pharmaceuticals Co., Ltd. (Japan) among others |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Lysosomal Storage Disorder Drugs Market Definition
Lysosomal storage disorder drugs refer to a category of pharmaceuticals specifically developed to treat Lysosomal Storage Disorders (LSDs), which are a group of rare genetic conditions caused by deficiencies in specific enzymes responsible for breaking down complex molecules within lysosomes. These drugs aim to address the underlying metabolic abnormalities associated with LSDs by either replacing the missing enzymes (enzyme replacement therapy), by enhancing the body's ability to produce the enzymes, or by modifying the substrates that accumulate due to enzyme deficiency (substrate reduction therapy). By improving enzymatic function or reducing the toxic build-up of substrates, these treatments can alleviate symptoms, slow disease progression, and ultimately improve the quality of life for individuals affected by these challenging conditions.
Lysosomal Storage Disorder Drugs Market Dynamics
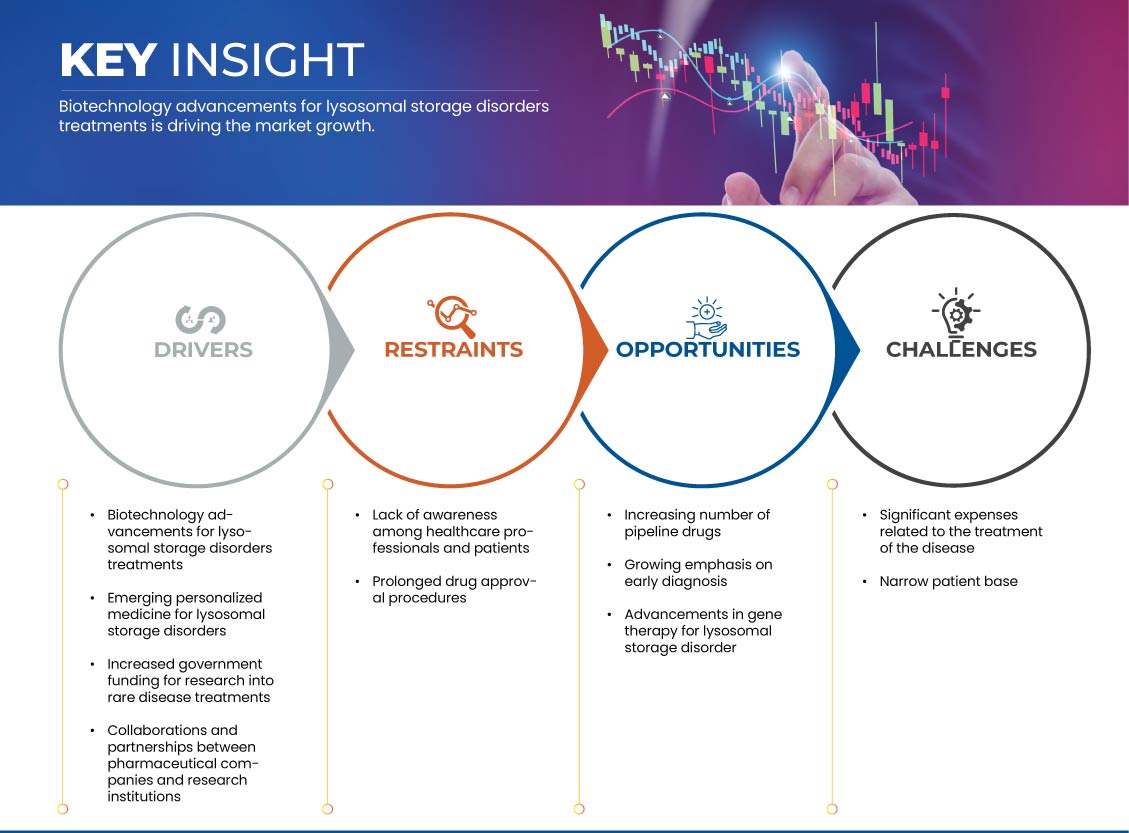
Drivers
- Emerging Personalized Medicine for Lysosomal Storage Disorders
Emerging personalized medicine is transforming the landscape of treatment for Lysosomal Storage Disorders (LSDs) by offering therapies specifically tailored to the individual genetic profiles of patients. LSDs are caused by genetic mutations that affect enzyme function, and these mutations can vary widely between patients. Personalized treatments involve analyzing a patient’s unique genetic makeup to develop a more targeted approach, optimizing the efficacy of therapies such as enzyme replacement or gene therapy. This precision allows for better matching of treatments with the patient's specific needs, minimizing side effects and enhancing therapeutic outcomes. As advancements in genetic testing and technology progress, healthcare providers are able to identify the most suitable interventions, potentially improving response rates and overall treatment success. The shift toward personalized medicine is accelerating the development of customized LSD treatments, which promise to revolutionize disease management. With these innovations, the global lysosomal storage disorders drug market is witnessing increased demand for more effective, individualized treatment options. Personalized medicine is thus a key driver of market growth, as it boosts treatment effectiveness and enhances patient outcomes, fostering greater confidence in therapeutic options.
For instance,
In February 2023, according to the article published by NCBI, Emerging personalized medicine uses an individual’s genetic profile to guide decisions related to the prevention, diagnosis, and treatment of diseases. This approach enables more precise and effective therapies for Lysosomal Storage Disorders (LSDs), tailoring treatments to specific genetic mutations. As personalized medicine becomes more prevalent, it drives the demand for targeted LSD therapies, fueling growth in the global lysosomal storage disorders drug market.
- Increased Government Funding for Research into Rare Disease Treatments
Government funding and grants for research into rare disease treatments are crucial for advancing the development of therapies for conditions such as Lysosomal Storage Disorders (LSDs). Rare diseases, often lacking commercial viability due to small patient populations, pose significant challenges in drug development. To address this, governments worldwide are increasingly allocating funds to incentivize research in these underserved areas. Financial support in the form of grants, subsidies, and tax credits helps pharmaceutical companies and research institutions overcome the high costs and risks associated with developing treatments for rare diseases. Furthermore, governments often fast-track regulatory processes for these treatments, recognizing the urgent need for solutions. By reducing the financial burden on researchers and companies, these funding mechanisms enable the exploration of innovative therapies, such as enzyme replacement and gene therapies that would otherwise face significant barriers to development.
For instance,
In August 2023, according to the article published by National Organization for Rare Diseases, The National Organization for Rare Disorders announced over USD 100,000 in grant funding for rare disease research, highlighting increased government support for advancing treatments. These funds enable researchers to explore innovative therapies for rare diseases like Lysosomal Storage Disorders (LSDs). Such financial backing accelerates drug development, driving growth in the global LSD drug market by fostering new treatment options.
Opportunities
- Increasing Number of Pipeline Drugs
The increasing number of drugs in the pipeline for Lysosomal Storage Disorders (LSDs) represents a significant opportunity for the global lysosomal storage disorders drugs market, potentially leading to improved treatment options and enhanced patient outcomes. As researchers and pharmaceutical companies enhance their understanding of these disorders, they are developing a diverse array of novel therapies, including gene therapies, substrate reduction therapies, and small molecule drugs. This expanded pipeline reflects a growing recognition of the unmet medical needs within the LSD community and promises to offer patients a wider variety of treatment modalities tailored to their specific conditions. The introduction of innovative therapies can enhance patient adherence to treatment, reduce disease burden, and ultimately improve the quality of life for individuals living with these disorders.
For instance,
In June 2024, Ultragenyx announced the Planning to file for accelerated approval of UX111 for the treatment of Sanfilippo Syndrome Type A (MPS IIIA). UX111 is a novel in vivo gene therapy in Phase 1/2/3 development for Sanfilippo syndrome type A (MPS IIIA), a rare fatal lysosomal storage disease with no approved treatment that primarily affects the central nervous system.
- Growing Emphasis on Early Diagnosis
The growing emphasis on early diagnosis of Lysosomal Storage Disorders (LSDs) presents a significant opportunity for the global lysosomal storage disorders drugs market by facilitating timely interventions that can greatly enhance patient outcomes. As healthcare systems place greater emphasis on early detection through improved screening programs, genetic testing, and advancements in diagnostic technologies, more patients are likely to be diagnosed at an earlier stage of their diseases. This early intervention allows for better management of symptoms but increases the potential efficacy of existing and emerging therapies. The result of this shift is a broader patient base that requires and can benefit from treatment options, ultimately driving demand within the lysosomal storage disorders drug market.
For instance,
In February 2023, according to an article, ‘Lysosomal storage disorders: from biology to the clinic with reference to India’, early diagnosis is the most critical part of the management of LSDs as it provides the opportunity for therapeutic intervention, precise genetic counselling, prenatal diagnosis and a better outcome for the patient.
Restraints/Challenges
- Lack of Awareness Among Healthcare Professionals and Patients
The lack of awareness among healthcare professionals and patients about Lysosomal Storage Disorders (LSDs) presents a significant barrier to early diagnosis, treatment, and effective management. Many healthcare providers, especially in regions with limited exposure to rare diseases, often fail to recognize the symptoms of LSDs, which are diverse and overlap with other more common conditions. This lack of knowledge leads to delayed diagnoses, misdiagnosis, and ineffective treatment, exacerbating the severity of the disease. For patients, especially those in resource-poor or underserved areas, the lack of awareness prevents early intervention, leaving them unaware of potential therapies. The complexity and rarity of LSDs further complicate the situation, as patients and healthcare professionals may not fully understand the importance of early treatment or the availability of specialized care options. Fewer patients are diagnosed and treated in time, leading to suboptimal clinical outcomes. This lack of awareness limits the demand for LSD-specific treatments and hinders the overall growth of the global lysosomal storage disorders drugs market.
For instance,
In January 2024, according to the article published by Wiley, there’s a significant diagnostic delay of around 15 years from symptom onset to diagnosis is common in adult LSD cases due to overlapping clinical phenotypes and varying severity. This delay highlights the lack of awareness among healthcare professionals, particularly those treating adult patients. Such restraints hinder early detection and timely treatment, restricting the growth of the global lysosomal storage disorders drugs market.
- Prolonged Drug Approval Procedures
Lengthy drug approval processes present a significant restraint for the global lysosomal storage disorder drugs market, slowing the introduction of much-needed therapies. The process for obtaining regulatory approval for new drugs, particularly for rare diseases like LSDs, involves numerous clinical trials, extensive documentation, and rigorous assessments by regulatory bodies such as the FDA and EMA. These approval timelines are often extended due to the need for a thorough evaluation of safety, efficacy, and potential long-term effects. In the case of LSDs, the rarity and complexity of these diseases add another layer of challenge, as there is a limited patient population for clinical trials, making it difficult to generate robust data. The need for specialized treatments and the lack of established standards for these rare diseases complicates the approval process. Consequently, drug developers face prolonged waiting periods, delays in bringing new treatments to market, and additional costs, which can be discouraging for pharmaceutical companies. As a result, the lengthy approval processes slow the availability of innovative therapies for patients, reducing market growth and hindering the accessibility of effective treatments for LSDs. This regulatory delay ultimately acts as a major restraint for the global lysosomal storage disorders drugs market.
For instance,
In August 2024, according to the article published by Drugs.com, The research, development, and approval process for drugs typically takes 12 to 15 years. This extended timeline delays the introduction of new therapies and increases costs for pharmaceutical companies. As a result, the lengthy drug approval process restrains the growth of the global LSD drug market by limiting the speed of treatment availability.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
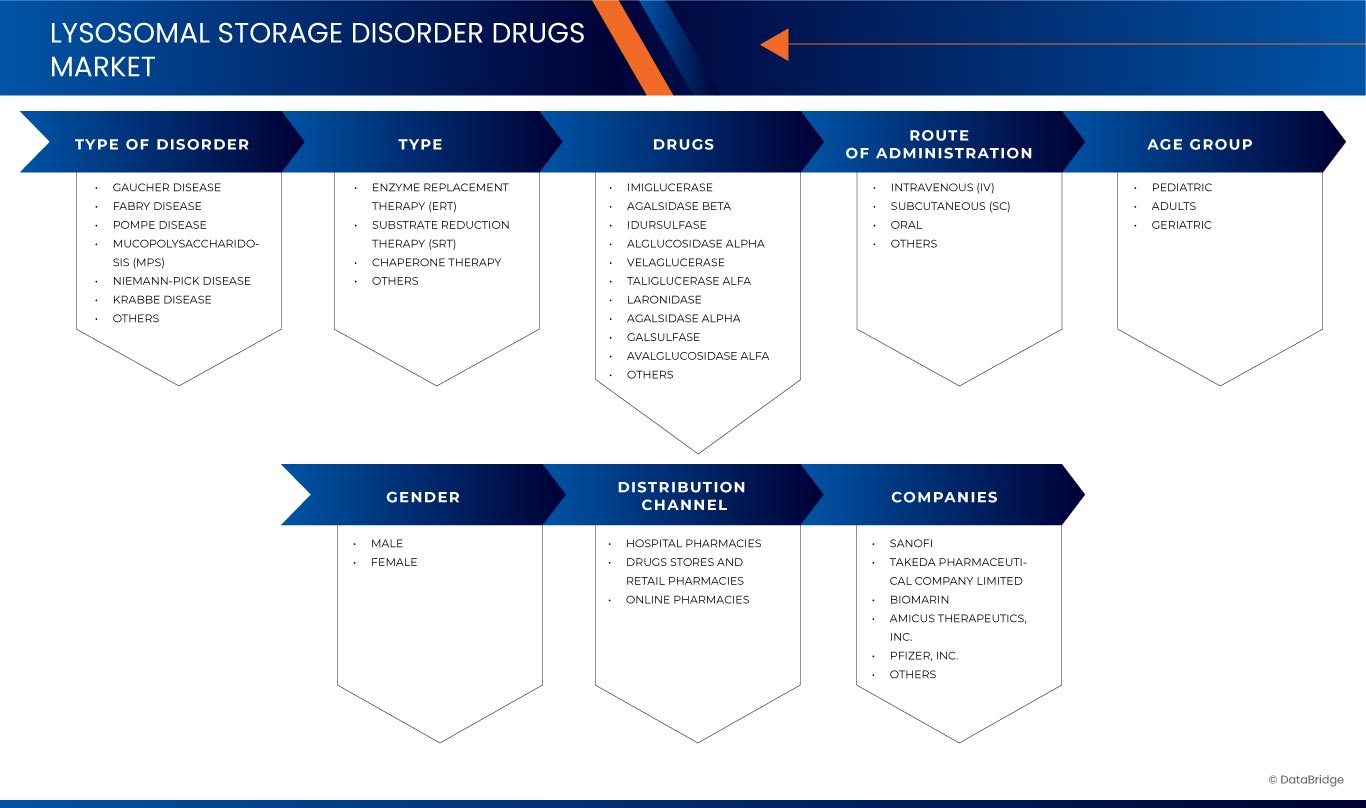
Lysosomal Storage Disorder Drugs Market Scope
The market is segmented on the basis of by type of disorder, type, drugs, route of administration, age group, gender, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type of Disorder
- Gaucher Disease
- Type 1
- Type 3
- Type 2
- Fabry Disease
- Pompe Disease
- Infantile-Onset Pompe
- Late-Onset Pompe
- Mucopolysaccharidosis (MPS)
- MPS I
- MPS II
- MPS IV
- MPS VI
- MPS III
- Niemann-Pick Disease
- Type C
- Type B
- Type A
- Krabbe Disease
- Others
Type
- Enzyme Replacement Therapy (ERT)
- Substrate Reduction Therapy (SRT)
- Chaperone Therapy
- Others
Drugs
- Imiglucerase
- Agalsidase Beta
- Idursulfase
- Alglucosidase Alpha
- Velaglucerase
- Taliglucerase Alfa
- Laronidase
- Agalsidase Alpha
- Galsulfase
- Avalglucosidase Alfa
- Others
Route of Administration
- Intravenous (IV)
- Subcutaneous (SC)
- Oral
- Others
Age Group
- Pediatric
- Adults
- Geriatric
Gender
- Male
- Female
Distribution Channel
- Hospital Pharmacies
- Drugs Stores and Retail Pharmacies
- Online Pharmacies
Lysosomal Storage Disorder Drugs Market Regional Analysis
The market is analyzed and market size insights and trends are provided by type of disorder, type, drugs, route of administration, age group, gender, and distribution channel as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, U.K., Italy, France, Spain, Russia, Switzerland, Turkey, Belgium, Netherlands, Denmark, Sweden, Poland, Norway, Finland, rest of Europe, Japan, China, South Korea, India, Singapore, Thailand, Indonesia, Malaysia, Philippines, Australia, New Zealand, Vietnam, Taiwan, rest of Asia-Pacific, Brazil, Argentina, rest of South America, South Africa, Egypt, Bahrain, United Arab Emirates, Kuwait, Oman, Qatar, Saudi Arabia, and rest of Middle East and Africa.
North America is expected to dominate the global lysosomal storage disorder drugs market due to its advanced healthcare infrastructure, high adoption of drugs, significant research and development investments, and a large patient population.
Asia-Pacific is expected to be the fastest growing due to increasing healthcare infrastructure, rising prevalence of lysosomal storage diseases, and growing awareness about advanced therapies.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Lysosomal Storage Disorder Drugs Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Lysosomal Storage Disorder Drugs Market Leaders Operating in the Market Are:
- Sanofi (France)
- BioMarin (U.S.)
- Pfizer Inc. (U.S.)
- Amicus Therapeutics, Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Orchard Therapeutics plc (U.K.)
- Spur Therapeutics (U.K.)
- Sangamo Therapeutics (U.S.)
- Protalix BioTherapeutics Inc. (Israel)
- Chiesi Farmaceutici S.p.A. (Italy)
- Forge Biologics (U.S.)
- Denali Therapeutics (U.S.)
- REGENXBIO INC. (U.S.)
- JCR Pharmaceuticals Co., Ltd. (Japan)
Latest Developments in Lysosomal Storage Disorder Drugs Market
- In October 2024, Amicus Therapeutics announced a settlement regarding its drug Galafold (migalastat), which treats Fabry disease. The settlement resolves ongoing patent disputes and confirms the continuation of the drug’s marketing without litigation interference. This agreement with multiple parties aims to provide stability for Galafold’s availability and development while protecting intellectual property rights
- In June 2024, Amicus Therapeutics was honored with the Prix Galien UK Award for its innovative treatment, Pombiliti (miglustat), for the management of Fabry disease. The award recognizes excellence in pharmaceutical innovation and emphasizes the impact of Pombiliti in improving the lives of patients with rare genetic conditions. This accolade highlights Amicus’ leadership in rare disease therapies
- In April 2024, Forge Biologics announced it would present nine times at the ASGCT 27th Annual Meeting in May 2024, including a late-breaking oral presentation and three technical sessions. Presentations will cover process development, molecular advancements, and clinical updates, including a significant clinical result for FBX-101 in Krabbe disease
- In November 2023, Chiesi Group has been reaccredited as a Great Place to Work-Certified organization across multiple regions, including Italy, Australia, the US, and others. With an 85% response rate from employees, Chiesi achieved an 83% overall satisfaction rate, reflecting its commitment to fostering a positive, inclusive, and collaborative work environment focused on employee well-being and growth
- In January 2024, Denali Therapeutics Inc., a biopharmaceutical company developing therapies to cross the blood-brain barrier for treating neurodegenerative and lysosomal storage diseases, announced progress and milestones for 2024. CEO Ryan Watts, Ph.D., highlighted these developments during a corporate presentation at the 42nd Annual J.P. Morgan Healthcare Conference on January 9th
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 SECONDARY SOURCES
2.1 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PORTER’S FIVE FORCES
4.2 PIPELINE ANALYSIS
5 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: REGULATIONS
5.1 REGULATORY AUTHORITIES IN NORTH AMERICA
5.2 REGULATORY AUTHORITIES IN SOUTH AMERICA
5.3 REGULATORY AUTHORITIES IN EUROPE
5.4 REGULATORY AUTHORITIES IN THE MIDDLE EAST AND AFRICA.
5.5 REGULATORY AUTHORITIES IN ASIA-PACIFIC.
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 BIOTECHNOLOGY ADVANCEMENTS FOR LYSOSOMAL STORAGE DISORDERS TREATMENTS
6.1.2 EMERGING PERSONALIZED MEDICINE FOR LYSOSOMAL STORAGE DISORDERS
6.1.3 INCREASED GOVERNMENT FUNDING FOR RESEARCH INTO RARE DISEASE TREATMENTS
6.1.4 COLLABORATIONS AND PARTNERSHIPS BETWEEN PHARMACEUTICAL COMPANIES AND RESEARCH INSTITUTIONS
6.2 RESTRAINTS
6.2.1 LACK OF AWARENESS AMONG HEALTHCARE PROFESSIONALS AND PATIENTS
6.2.2 PROLONGED DRUG APPROVAL PROCEDURES
6.3 OPPORTUNITIES
6.3.1 INCREASING NUMBER OF PIPELINE DRUGS
6.3.2 GROWING EMPHASIS ON EARLY DIAGNOSIS
6.3.3 ADVANCEMENTS IN GENE THERAPY FOR THE TREATMENT OF LYSOSOMAL STORAGE DISORDERS
6.4 CHALLENGES
6.4.1 SIGNIFICANT EXPENSES RELATED TO THE TREATMENT OF THE DISEASE
6.4.2 NARROW PATIENT BASE SUFFERING FROM LYSOSOMAL STORAGE DISORDERS
7 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER
7.1 OVERVIEW
7.2 GAUCHER DISEASE
7.2.1 TYPE 1
7.2.2 TYPE 3
7.2.3 TYPE 2
7.3 FABRY DISEASE
7.4 POMPE DISEASE
7.4.1 INFANTILE-ONSET POMPE
7.4.2 LATE-ONSET POMPE
7.5 MUCOPOLYSACCHARIDOSIS (MPS)
7.5.1 MPS I
7.5.2 MPS II
7.5.3 MPS IV
7.5.4 MPS VI
7.5.5 MPS III
7.6 NIEMANN-PICK DISEASE
7.6.1 TYPE C
7.6.2 TYPE B
7.6.3 TYPE A
7.7 KRABBE DISEASE
7.8 OTHERS
8 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS
8.1 OVERVIEW
8.2 IMIGLUCERASE
8.3 AGALSIDASE BETA
8.4 IDURSULFASE
8.5 ALGLUCOSIDASE ALPHA
8.6 VELAGLUCERASE
8.7 TALIGLUCERASE ALFA
8.8 LARONIDASE
8.9 AGALSIDASE ALPHA
8.1 GALSULFASE
8.11 AVALGLUCOSIDASE ALFA
8.12 OTHERS
9 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE
9.1 OVERVIEW
9.2 ENZYME REPLACEMENT THERAPY (ERT)
9.3 SUBSTRATE REDUCTION THERAPY (SRT)
9.4 CHAPERONE THERAPY
9.5 OTHERS
10 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER
10.1 OVERVIEW
10.2 MALE
10.3 FEMALE
11 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 PEDIATRIC
11.3 ADULT
11.4 GERIATRIC
12 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 HOSPITAL PHARMACIES
12.3 DRUGS STORES AND RETAIL PHARMACIES
12.4 ONLINE PHARMACIES
13 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 INTRAVENOUS (IV)
13.3 SUBCUTANEOUS (SC)
13.4 ORAL
13.5 OTHERS
14 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION
14.1 OVERVIEW
14.2 NORTH AMERICA
14.2.1 U.S.
14.2.2 CANADA
14.2.3 MEXICO
14.3 EUROPE
14.3.1 GERMANY
14.3.2 UNITED KINGDOM
14.3.3 FRANCE
14.3.4 ITALY
14.3.5 SPAIN
14.3.6 RUSSIA
14.3.7 SWITZERLAND
14.3.8 TURKEY
14.3.9 BELGIUM
14.3.10 NETHERLANDS
14.3.11 DENMARK
14.3.12 SWEDEN
14.3.13 POLAND
14.3.14 NORWAY
14.3.15 FINLAND
14.3.16 REST OF EUROPE
14.4 ASIA-PACIFIC
14.4.1 CHINA
14.4.2 JAPAN
14.4.3 INDIA
14.4.4 SOUTH KOREA
14.4.5 AUSTRALIA
14.4.6 THAILAND
14.4.7 SINGAPORE
14.4.8 INDONESIA
14.4.9 MALAYSIA
14.4.10 PHILIPPINES
14.4.11 TAIWAN
14.4.12 NEW ZEALAND
14.4.13 VIETNAM
14.4.14 REST OF ASIA-PACIFIC
14.5 MIDDLE EAST AND AFRICA
14.5.1 SAUDI ARABIA
14.5.2 UNITED ARAB EMIRATES (UAE)
14.5.3 SOUTH AFRICA
14.5.4 EGYPT
14.5.5 QATAR
14.5.6 KUWAIT
14.5.7 OMAN
14.5.8 BAHRAIN
14.5.9 REST OF MIDDLE EAST AND AFRICA
14.6 SOUTH AMERICA
14.6.1 BRAZIL
14.6.2 ARGENTINA
14.6.3 REST OF SOUTH AMERICA
15 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: GLOBAL
15.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
15.3 COMPANY SHARE ANALYSIS: EUROPE
15.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
16 SWOT ANALYSIS
17 COMPANY PROFILES
17.1 SANOFI
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 BIOMARIN
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.3 PFIZER INC.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENT
17.4 TAKEDA PHARMACEUTICAL COMPANY LIMITED
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 AMICUS THERAPEUTIC, INC.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 CHIESI FARMACEUTICI S.P.A.
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENT
17.7 DENALI THERAPEUTICS
17.7.1 COMPANY SNAPSHOT
17.7.2 REVENUE ANALYSIS
17.7.3 PIPELINE PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENT
17.8 FORGE BIOLOGICS
17.8.1 COMPANY SNAPSHOT
17.8.2 PIPELINE PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENT
17.9 JCR PHARMACEUTICALS CO., LTD.
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PINELINE PRODUCT PORTFOLIO
17.9.4 PRODUCT PORTFOLIO
17.9.5 RECENT DEVELOPMENT
17.1 ORCHARD THERAPEUTICS PLC
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.11 PROTALIX BIOTHERAPEUTICS
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PIPELINE PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENT
17.12 REGENXBIO INC.
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PINELINE PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENT
17.13 SANGAMO THERAPEUTICS
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 SPUR THERAPEUTICS
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.15 ULTRAGENYX PHARMACEUTICAL INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
List of Table
TABLE 1 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 2 GLOBAL GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 3 GLOBAL GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 4 GLOBAL FABRY DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 5 GLOBAL POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 6 GLOBAL POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 7 GLOBAL MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 8 GLOBAL MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 9 GLOBAL NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 10 GLOBAL NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 11 GLOBAL KRABBE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 12 GLOBAL OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 13 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 14 GLOBAL IMIGLUCERASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 15 GLOBAL AGALSIDASE BETA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 16 GLOBAL IDURSULFASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 17 GLOBAL ALGLUCOSIDASE ALPHA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 18 GLOBAL VELAGLUCERASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 19 GLOBAL TALIGLUCERASE ALFA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 20 GLOBAL LARONIDASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 21 GLOBAL AGALSIDASE ALPHA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 22 GLOBAL GALSULFASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 23 GLOBAL AVALGLUCOSIDASE ALFA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 24 GLOBAL OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 25 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 26 GLOBAL ENZYME REPLACEMENT THERAPY (ERT) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 27 GLOBAL SUBSTRATE REDUCTION THERAPY (SRT) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 28 GLOBAL CHAPERONE THERAPY IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 29 GLOBAL OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 30 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 31 GLOBAL MALE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 32 GLOBAL FEMALE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 33 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 34 GLOBAL PEDIATRIC IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 35 GLOBAL ADULT IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 36 GLOBAL GERIATRIC IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 37 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 38 GLOBAL HOSPITAL PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 39 GLOBAL DRUGS STORES AND RETAIL PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 40 GLOBAL ONLINE PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 41 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 42 GLOBAL INTRAVENOUS (IV) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 43 GLOBAL SUBCUTANEOUS (SC) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 44 GLOBAL ORAL IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 45 GLOBAL OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 46 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY COUNTRY, 2018-2032 (USD MILLION)
TABLE 47 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 48 NORTH AMERICA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 49 NORTH AMERICA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 50 NORTH AMERICA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 51 NORTH AMERICA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 52 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 53 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 54 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 55 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 56 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 57 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 58 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 59 U.S. GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 60 U.S. POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 61 U.S. MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 62 U.S. NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 63 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 64 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 65 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 66 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 67 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 68 U.S. LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 69 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 70 CANADA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 71 CANADA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 72 CANADA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 73 CANADA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 74 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 75 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 76 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 77 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 78 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 79 CANADA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 80 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 81 MEXICO GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 82 MEXICO POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 83 MEXICO MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 84 MEXICO NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 85 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 86 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 87 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 88 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 89 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 90 MEXICO LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 91 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY COUNTRY, 2018-2032 (USD MILLION)
TABLE 92 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 93 EUROPE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 94 EUROPE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 95 EUROPE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 96 EUROPE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 97 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 98 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 99 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 100 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 101 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 102 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 103 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 104 GERMANY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 105 GERMANY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 106 GERMANY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 107 GERMANY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 108 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 109 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 110 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 111 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 112 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 113 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 114 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 115 UNITED KINGDOM GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 116 UNITED KINGDOM POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 117 UNITED KINGDOM MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 118 UNITED KINGDOM NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 119 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 120 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 121 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 122 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 123 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 124 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 125 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 126 FRANCE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 127 FRANCE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 128 FRANCE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 129 FRANCE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 130 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 131 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 132 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 133 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 134 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 135 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 136 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 137 ITALY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 138 ITALY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 139 ITALY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 140 ITALY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 141 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 142 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 143 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 144 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 145 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 146 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 147 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 148 SPAIN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 149 SPAIN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 150 SPAIN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 151 SPAIN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 152 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 153 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 154 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 155 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 156 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 157 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 158 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 159 RUSSIA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 160 RUSSIA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 161 RUSSIA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 162 RUSSIA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 163 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 164 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 165 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 166 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 167 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 168 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 169 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 170 SWITZERLAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 171 SWITZERLAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 172 SWITZERLAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 173 SWITZERLAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 174 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 175 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 176 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 177 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 178 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 179 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 180 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 181 TURKEY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 182 TURKEY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 183 TURKEY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 184 TURKEY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 185 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 186 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 187 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 188 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 189 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 190 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 191 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 192 BELGIUM GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 193 BELGIUM POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 194 BELGIUM MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 195 BELGIUM NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 196 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 197 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 198 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 199 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 200 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 201 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 202 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 203 NETHERLANDS GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 204 NETHERLANDS POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 205 NETHERLANDS MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 206 NETHERLANDS NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 207 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 208 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 209 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 210 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 211 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 212 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 213 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 214 DENMARK GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 215 DENMARK POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 216 DENMARK MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 217 DENMARK NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 218 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 219 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 220 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 221 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 222 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 223 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 224 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 225 SWEDEN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 226 SWEDEN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 227 SWEDEN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 228 SWEDEN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 229 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 230 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 231 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 232 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 233 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 234 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 235 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 236 POLAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 237 POLAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 238 POLAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 239 POLAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 240 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 241 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 242 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 243 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 244 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 245 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 246 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 247 NORWAY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 248 NORWAY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 249 NORWAY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 250 NORWAY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 251 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 252 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 253 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 254 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 255 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 256 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 257 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 258 FINLAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 259 FINLAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 260 FINLAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 261 FINLAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 262 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 263 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 264 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 265 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 266 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 267 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 268 REST OF EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 269 ASIA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY COUNTRY, 2018-2032 (USD MILLION)
TABLE 270 ASIA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 271 ASIA-PACIFIC GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 272 ASIA-PACIFIC POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 273 ASIA-PACIFIC MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 274 ASIA-PACIFIC NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 275 ASIA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 276 ASIA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 277 ASIA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 278 ASIA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 279 ASIA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 280 ASIA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 281 CHINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 282 CHINA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 283 CHINA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 284 CHINA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 285 CHINA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 286 CHINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 287 CHINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 288 CHINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 289 CHINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 290 CHINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 291 CHINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 292 JAPAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 293 JAPAN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 294 JAPAN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 295 JAPAN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 296 JAPAN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 297 JAPAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 298 JAPAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 299 JAPAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 300 JAPAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 301 JAPAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 302 JAPAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 303 INDIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 304 INDIA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 305 INDIA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 306 INDIA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 307 INDIA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 308 INDIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 309 INDIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 310 INDIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 311 INDIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 312 INDIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 313 INDIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 314 SOUTH KOREA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 315 SOUTH KOREA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 316 SOUTH KOREA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 317 SOUTH KOREA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 318 SOUTH KOREA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 319 SOUTH KOREA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 320 SOUTH KOREA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 321 SOUTH KOREA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 322 SOUTH KOREA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 323 SOUTH KOREA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 324 SOUTH KOREA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 325 AUSTRALIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 326 AUSTRALIA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 327 AUSTRALIA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 328 AUSTRALIA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 329 AUSTRALIA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 330 AUSTRALIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 331 AUSTRALIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 332 AUSTRALIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 333 AUSTRALIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 334 AUSTRALIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 335 AUSTRALIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 336 THAILAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 337 THAILAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 338 THAILAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 339 THAILAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 340 THAILAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 341 THAILAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 342 THAILAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 343 THAILAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 344 THAILAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 345 THAILAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 346 THAILAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 347 SINGAPORE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 348 SINGAPORE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 349 SINGAPORE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 350 SINGAPORE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 351 SINGAPORE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 352 SINGAPORE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 353 SINGAPORE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 354 SINGAPORE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 355 SINGAPORE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 356 SINGAPORE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 357 SINGAPORE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 358 INDONESIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 359 INDONESIA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 360 INDONESIA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 361 INDONESIA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 362 INDONESIA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 363 INDONESIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 364 INDONESIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 365 INDONESIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 366 INDONESIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 367 INDONESIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 368 INDONESIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 369 MALAYSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 370 MALAYSIA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 371 MALAYSIA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 372 MALAYSIA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 373 MALAYSIA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 374 MALAYSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 375 MALAYSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 376 MALAYSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 377 MALAYSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 378 MALAYSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 379 MALAYSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 380 PHILIPPINES LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 381 PHILIPPINES GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 382 PHILIPPINES POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 383 PHILIPPINES MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 384 PHILIPPINES NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 385 PHILIPPINES LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 386 PHILIPPINES LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 387 PHILIPPINES LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 388 PHILIPPINES LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 389 PHILIPPINES LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 390 PHILIPPINES LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 391 TAIWAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 392 TAIWAN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 393 TAIWAN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 394 TAIWAN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 395 TAIWAN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 396 TAIWAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 397 TAIWAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 398 TAIWAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 399 TAIWAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 400 TAIWAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 401 TAIWAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 402 NEW ZEALAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 403 NEW ZEALAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 404 NEW ZEALAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 405 NEW ZEALAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 406 NEW ZEALAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 407 NEW ZEALAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 408 NEW ZEALAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 409 NEW ZEALAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 410 NEW ZEALAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 411 NEW ZEALAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 412 NEW ZEALAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 413 VIETNAM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 414 VIETNAM GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 415 VIETNAM POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 416 VIETNAM MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 417 VIETNAM NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 418 VIETNAM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 419 VIETNAM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 420 VIETNAM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 421 VIETNAM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 422 VIETNAM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 423 VIETNAM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 424 REST OF ASIA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 425 MIDDLE EAST AND AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY COUNTRY, 2018-2032 (USD MILLION)
TABLE 426 MIDDLE EAST AND AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 427 MIDDLE EAST AND AFRICA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 428 MIDDLE EAST AND AFRICA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 429 MIDDLE EAST AND AFRICA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 430 MIDDLE EAST AND AFRICA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 431 MIDDLE EAST AND AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 432 MIDDLE EAST AND AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 433 MIDDLE EAST AND AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 434 MIDDLE EAST AND AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 435 MIDDLE EAST AND AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 436 MIDDLE EAST AND AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 437 SAUDI ARABIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 438 SAUDI ARABIA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 439 SAUDI ARABIA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 440 SAUDI ARABIA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 441 SAUDI ARABIA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 442 SAUDI ARABIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 443 SAUDI ARABIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 444 SAUDI ARABIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 445 SAUDI ARABIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 446 SAUDI ARABIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 447 SAUDI ARABIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 448 UNITED ARAB EMIRATES (UAE) LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 449 UNITED ARAB EMIRATES (UAE) GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 450 UNITED ARAB EMIRATES (UAE) POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 451 UNITED ARAB EMIRATES (UAE) MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 452 UNITED ARAB EMIRATES (UAE) NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 453 UNITED ARAB EMIRATES (UAE) LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 454 UNITED ARAB EMIRATES (UAE) LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 455 UNITED ARAB EMIRATES (UAE) LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 456 UNITED ARAB EMIRATES (UAE) LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 457 UNITED ARAB EMIRATES (UAE) LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 458 UNITED ARAB EMIRATES (UAE) LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 459 SOUTH AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 460 SOUTH AFRICA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 461 SOUTH AFRICA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 462 SOUTH AFRICA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 463 SOUTH AFRICA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 464 SOUTH AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 465 SOUTH AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 466 SOUTH AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 467 SOUTH AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 468 SOUTH AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 469 SOUTH AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 470 EGYPT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 471 EGYPT GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 472 EGYPT POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 473 EGYPT MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 474 EGYPT NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 475 EGYPT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 476 EGYPT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 477 EGYPT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 478 EGYPT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 479 EGYPT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 480 EGYPT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 481 QATAR LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 482 QATAR GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 483 QATAR POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 484 QATAR MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 485 QATAR NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 486 QATAR LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 487 QATAR LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 488 QATAR LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 489 QATAR LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 490 QATAR LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 491 QATAR LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 492 KUWAIT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 493 KUWAIT GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 494 KUWAIT POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 495 KUWAIT MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 496 KUWAIT NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 497 KUWAIT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 498 KUWAIT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 499 KUWAIT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 500 KUWAIT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 501 KUWAIT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 502 KUWAIT LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 503 OMAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 504 OMAN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 505 OMAN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 506 OMAN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 507 OMAN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 508 OMAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 509 OMAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 510 OMAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 511 OMAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 512 OMAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 513 OMAN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 514 BAHRAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 515 BAHRAIN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 516 BAHRAIN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 517 BAHRAIN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 518 BAHRAIN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 519 BAHRAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 520 BAHRAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 521 BAHRAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 522 BAHRAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 523 BAHRAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 524 BAHRAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 525 REST OF MIDDLE EAST AND AFRICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 526 SOUTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY COUNTRY, 2018-2032 (USD MILLION)
TABLE 527 SOUTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 528 SOUTH AMERICA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 529 SOUTH AMERICA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 530 SOUTH AMERICA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 531 SOUTH AMERICA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 532 SOUTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 533 SOUTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 534 SOUTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 535 SOUTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 536 SOUTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 537 SOUTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 538 BRAZIL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 539 BRAZIL GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 540 BRAZIL POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 541 BRAZIL MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 542 BRAZIL NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 543 BRAZIL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 544 BRAZIL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 545 BRAZIL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 546 BRAZIL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 547 BRAZIL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 548 BRAZIL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 549 ARGENTINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 550 ARGENTINA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 551 ARGENTINA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 552 ARGENTINA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 553 ARGENTINA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 554 ARGENTINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 555 ARGENTINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 556 ARGENTINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 557 ARGENTINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 558 ARGENTINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 559 ARGENTINA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 560 REST OF SOUTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
List of Figure
FIGURE 1 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SEGMENTATION
FIGURE 2 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: MULTIVARIATE MODELLING
FIGURE 7 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SEGMENTATION
FIGURE 10 EXECUTIVE SUMMARY
FIGURE 11 SEVEN SEGMENTS COMPRISE THE GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE GLOBAL LYSOSOMAL STORAGE DISORDER MARKET AND ASIA PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 14 BIOTECHNOLOGY ADVANCEMENTS FOR LYSOSOMAL STORAGE DISORDER TREATMENTS IS EXPECTED TO DRIVE THE GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET IN THE FORECAST PERIOD
FIGURE 15 GAUCHER DISEASE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET IN 2025 & 2032
FIGURE 16 ASIA PACIFIC IS THE FASTEST GROWING MARKET FOR THE GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET
FIGURE 17 PESTEL ANALYSIS
FIGURE 18 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET
FIGURE 19 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, 2024
FIGURE 20 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, 2025-2032 (USD MILLION)
FIGURE 21 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, CAGR (2025-2032)
FIGURE 22 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, LIFELINE CURVE
FIGURE 23 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, 2024
FIGURE 24 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, 2025-2032 (USD MILLION)
FIGURE 25 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, CAGR (2025-2032)
FIGURE 26 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, LIFELINE CURVE
FIGURE 27 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, 2024
FIGURE 28 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, 2025-2032 (USD MILLION)
FIGURE 29 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 30 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 31 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, 2024
FIGURE 32 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, 2025-2032 (USD MILLION)
FIGURE 33 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, CAGR (2025-2032)
FIGURE 34 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, LIFELINE CURVE
FIGURE 35 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, 2024
FIGURE 36 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, 2025-2032 (USD MILLION)
FIGURE 37 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, CAGR (2025-2032)
FIGURE 38 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 39 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 40 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD MILLION)
FIGURE 41 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 42 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2024
FIGURE 44 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2025-2032 (USD MILLION)
FIGURE 45 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2025-2032)
FIGURE 46 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 47 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SNAPSHOT 2024
FIGURE 48 GLOBAL LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY SHARE 2024 (%)
FIGURE 49 NORTH AMERICA LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY SHARE 2024 (%)
FIGURE 50 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY SHARE 2024 (%)
FIGURE 51 AISA-PACIFIC LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY SHARE 2024 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.