Global Malaria Diagnostics Market
Market Size in USD Million
CAGR :
% 
 USD
796.67 Million
USD
1,172.57 Million
2025
2033
USD
796.67 Million
USD
1,172.57 Million
2025
2033
| 2026 –2033 | |
| USD 796.67 Million | |
| USD 1,172.57 Million | |
|
|
|
|
Malaria Diagnostics Market Size
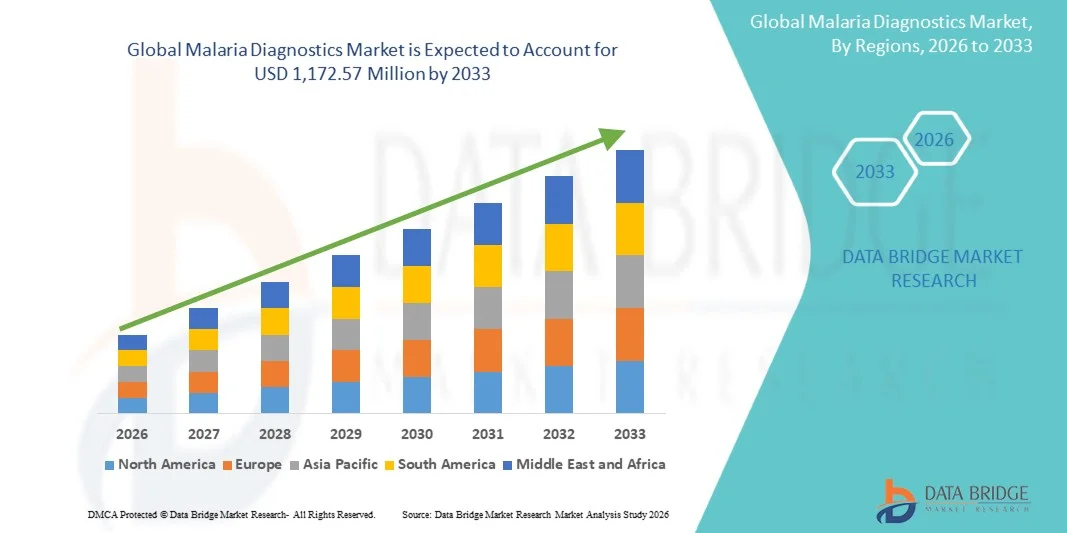
- The global malaria diagnostics market size was valued at USD 796.67 million in 2025 and is expected to reach USD 1,172.57 million by 2033, at a CAGR of 4.95% during the forecast period
- The market growth is largely fueled by the increasing prevalence of malaria in endemic regions and the growing emphasis on early and accurate detection through advanced diagnostic technologies, including rapid diagnostic tests (RDTs) and molecular diagnostics
- Furthermore, rising government initiatives, funding from global health organizations, and growing awareness about effective malaria management are driving demand for reliable, fast, and cost-effective diagnostic solutions. These converging factors are accelerating the adoption of malaria diagnostics, thereby significantly boosting the industry's growth
Malaria Diagnostics Market Analysis
- Malaria diagnostics, encompassing rapid diagnostic tests (RDTs), microscopy, and molecular testing methods, are increasingly critical for early detection and effective management of malaria infections in both endemic and non-endemic regions due to their accuracy, speed, and ease of use
- The escalating demand for malaria diagnostics is primarily fueled by the growing prevalence of malaria, rising government and NGO initiatives for malaria control, and the increasing emphasis on timely diagnosis to reduce disease burden and mortality
- Africa & Middle East dominated the malaria diagnostics market with the largest revenue share of 89.20% in 2025, characterized by high malaria incidence, extensive public health programs, and strong support from international health organizations, driving widespread adoption of rapid and point-of-care diagnostic solutions
- Asia-Pacific is expected to be the fastest growing region in the malaria diagnostics market during the forecast period due to expanding healthcare infrastructure, rising public health awareness, and increasing investments in malaria control initiatives
- Rapid diagnostic tests (RDTs) segment dominated the malaria diagnostics market with a market share of 68.3% in 2025, driven by their affordability, ease of deployment in remote areas, and ability to provide quick and reliable results without the need for specialized laboratory facilities
Report Scope and Malaria Diagnostics Market Segmentation
|
Attributes |
Malaria Diagnostics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Malaria Diagnostics Market Trends
“Advancements in Rapid and Point-of-Care Diagnostic Technologies”
- A significant and accelerating trend in the global malaria diagnostics market is the development and adoption of rapid diagnostic tests (RDTs) and point-of-care molecular diagnostics, enabling faster, more accurate detection in both endemic and non-endemic regions
- For instance, the CareStart™ Malaria RDT provides reliable results within 15–20 minutes, allowing healthcare providers to initiate treatment promptly without requiring complex laboratory infrastructure
- Integration of diagnostics with digital health platforms allows real-time reporting of malaria cases, improving outbreak tracking and intervention efficiency. For instance, some Abbott molecular diagnostic solutions are designed to upload test results directly to national health databases for timely epidemiological insights
- These intelligent diagnostic systems enhance accessibility and usability for healthcare workers in remote or resource-limited areas, reducing reliance on conventional microscopy and streamlining patient management
- The growing emphasis on user-friendly, rapid, and accurate malaria diagnostics is reshaping healthcare expectations, prompting companies such as SD Bioline to introduce AI-assisted RDT readers for improved accuracy and automated reporting
- The demand for advanced malaria diagnostic tools is rising rapidly across both public health programs and private healthcare sectors, as timely detection and treatment are critical to reducing morbidity and mortality
- Emerging multiplex diagnostic platforms that can simultaneously detect malaria and other co-infections are gaining traction, improving overall disease management
- Increasing collaborations between diagnostic manufacturers and public health agencies to deploy mobile testing units in remote regions are further accelerating adoption
Malaria Diagnostics Market Dynamics
Driver
“Rising Prevalence of Malaria and Strengthened Public Health Initiatives”
- The increasing incidence of malaria in endemic regions, combined with robust government and NGO programs to control and eliminate the disease, is a significant driver of demand for advanced diagnostics
- For instance, in March 2025, WHO-supported initiatives in Africa introduced nationwide RDT deployment campaigns to improve early detection and case management, boosting market adoption
- As healthcare systems prioritize accurate and timely diagnosis, rapid and point-of-care tests allow faster treatment initiation and better resource allocation, providing a compelling reason to replace traditional methods
- Furthermore, rising investments in healthcare infrastructure and growing awareness about malaria prevention and early diagnosis are integrating diagnostics into broader public health strategies
- The convenience of quick, reliable testing without extensive laboratory facilities, along with scalable deployment in high-risk regions, is propelling the adoption of malaria diagnostics across both urban and rural settings
- Increasing adoption of digital reporting tools for diagnostics enables faster epidemiological response, strengthening public health monitoring and intervention
- Growing support from global funding organizations and public-private partnerships is facilitating wider distribution of malaria diagnostic kits in low-income and high-risk regions
Restraint/Challenge
“Infrastructure Limitations and Cost Constraints”
- Limited healthcare infrastructure, particularly in rural and remote regions, poses a significant challenge to the widespread adoption of advanced malaria diagnostics. Resource-poor settings may lack trained personnel, reliable power, or proper storage conditions for test kits
- For instance, reports from rural sub-Saharan Africa indicate that inconsistent supply chains and lack of trained technicians delay timely diagnostic testing, reducing market penetration
- Addressing these limitations requires investment in portable, easy-to-use diagnostic solutions, robust supply chains, and training programs to ensure accurate and efficient testing
- In addition, high costs associated with advanced molecular diagnostic kits compared to basic RDTs can hinder adoption, especially in low-income countries or government-funded programs
- Overcoming these challenges through affordable, scalable diagnostics, mobile testing solutions, and public-private partnerships will be vital for sustained growth in the malaria diagnostics market
- Regulatory hurdles and variations in approval processes across different regions can delay the introduction of new diagnostic technologies, limiting market expansion
- Dependence on continuous supply of reagents and test kits, particularly during outbreaks, creates logistical challenges that may hinder timely testing and impact market growth
Malaria Diagnostics Market Scope
The market is segmented on the basis of technology and end user.
- By Technology
On the basis of technology, the malaria diagnostics market is segmented into microscopy, rapid diagnostics tests (RDTs), and molecular diagnostics tests. The RDTs segment dominated the market with the largest market revenue share of 68.3% in 2025, driven by their affordability, ease of use, and rapid results without requiring specialized laboratory infrastructure. RDTs are widely deployed in remote and resource-limited regions, allowing healthcare workers to diagnose malaria at the point-of-care. Their portability, minimal training requirements, and ability to provide reliable results within 15–20 minutes make them the preferred choice for large-scale malaria control programs. Governments and NGOs often rely on RDTs for mass screening campaigns and routine surveillance. The segment also benefits from continuous technological improvements, such as improved sensitivity and the introduction of AI-assisted readers for enhanced accuracy. These factors collectively reinforce RDTs as the backbone of malaria diagnostics in endemic regions.
The molecular diagnostics segment is anticipated to witness the fastest growth rate during 2026–2033, fueled by increasing demand for highly sensitive and specific tests capable of detecting low parasitemia and mixed infections. Molecular diagnostics, including PCR-based methods, allow precise detection of malaria even in asymptomatic carriers, which is critical for elimination programs. Rising investments in public health infrastructure and increasing availability of automated molecular testing platforms in hospitals and research centers further accelerate adoption. Molecular tests are also gaining traction in urban healthcare settings where accuracy and confirmatory diagnosis are prioritized. Their integration with digital reporting systems enables real-time epidemiological monitoring, boosting their significance in modern malaria control strategies.
- By End User
On the basis of end user, the malaria diagnostics market is segmented into hospitals, clinics, and community healthcare. The hospitals segment dominated the market in 2025, driven by the presence of advanced laboratory facilities, skilled personnel, and access to high-end diagnostic technologies such as molecular testing. Hospitals often handle severe or complicated malaria cases, requiring precise diagnosis to guide treatment and prevent fatalities. They also serve as referral centers for regional healthcare networks, increasing their reliance on both RDTs and molecular diagnostics. Government and private hospitals frequently participate in malaria surveillance programs, boosting the demand for integrated diagnostic solutions. In addition, hospitals adopt diagnostics with automated reporting features to enhance patient management and track outbreak trends efficiently. High patient volumes, centralized testing, and better resource allocation contribute to hospitals maintaining a dominant position in the market.
The community healthcare segment is expected to witness the fastest growth rate during 2026–2033, driven by expanding outreach programs in rural and underserved regions. Community health centers, mobile testing units, and local clinics increasingly deploy RDTs and portable molecular devices to facilitate early detection and treatment. Government and NGO initiatives promoting universal access to malaria diagnostics enhance this segment’s growth potential. The convenience, low cost, and rapid turnaround of point-of-care tests allow community healthcare providers to manage large populations efficiently. Integration of diagnostics with mobile health reporting systems further improves surveillance and outbreak management, making community healthcare an essential growth driver in the malaria diagnostics market.
Malaria Diagnostics Market Regional Analysis
- Africa & Middle East dominated the malaria diagnostics market with the largest revenue share of 89.20% in 2025, characterized by high malaria incidence, extensive public health programs, and strong support from international health organizations, driving widespread adoption of rapid and point-of-care diagnostic solutions
- Healthcare providers in the region highly value the accessibility, affordability, and rapid results offered by RDTs and point-of-care diagnostic solutions, which enable early detection and timely treatment in both rural and urban areas
- This widespread adoption is further supported by increasing investments in public health infrastructure, growing awareness of malaria prevention, and the integration of diagnostics into national disease surveillance programs, establishing malaria diagnostics as a critical component of healthcare delivery in the region
The Nigeria Malaria Diagnostics Market Insight
The Nigeria malaria diagnostics market captured a significant revenue share in 2025, driven by the country’s high malaria burden and widespread implementation of rapid diagnostic tests (RDTs) across urban and rural healthcare centers. Public health initiatives and government-led malaria control programs are expanding access to affordable, point-of-care testing solutions. Healthcare providers prioritize early detection and treatment, with molecular diagnostics increasingly used in hospitals for severe cases. International funding and technical support are strengthening supply chains and distribution networks. Mobile testing units and community health campaigns further enhance diagnostic coverage. The focus on reliable, accessible, and rapid testing solutions is accelerating market growth.
Kenya Malaria Diagnostics Market Insight
The Kenya malaria diagnostics market is witnessing strong growth due to the prevalence of malaria in endemic regions and government initiatives promoting early detection and treatment. RDTs are widely deployed in community health centers and rural clinics, providing quick and reliable results. Hospitals are increasingly adopting molecular diagnostics for accurate species identification and monitoring of high-risk populations. Public-private partnerships and international aid programs are improving diagnostic infrastructure and outreach. Digital reporting systems and mobile health initiatives are enhancing disease surveillance. The growing awareness of malaria prevention and treatment drives the demand for advanced diagnostic solutions.
Asia-Pacific Malaria Diagnostics Market Insight
The Asia-Pacific malaria diagnostics market is poised to grow at the fastest CAGR during the forecast period of 2026 to 2033, fueled by expanding healthcare infrastructure, rising public health awareness, and government initiatives aimed at malaria control and elimination. Countries such as India, Indonesia, and Thailand are witnessing increased adoption of RDTs and molecular diagnostics across both urban and rural healthcare settings. Integration with digital health platforms and mobile testing units is improving early detection and monitoring. Public-private partnerships and funding from global health organizations are further supporting market expansion. The focus on accessible, reliable, and cost-effective diagnostics is accelerating adoption across the region.
India Malaria Diagnostics Market Insight
The India malaria diagnostics market accounted for the largest revenue share in Asia-Pacific in 2025, driven by high malaria prevalence, extensive public health programs, and rapid adoption of RDTs and molecular diagnostics. Community health centers, urban hospitals, and mobile testing units are increasingly equipped with point-of-care diagnostic tools. Government initiatives and malaria elimination programs support widespread testing coverage. Partnerships with local manufacturers and international organizations are strengthening supply chains. Early detection and reliable diagnostic methods are improving treatment outcomes. Rising awareness and accessibility continue to propel market growth.
Indonesia Malaria Diagnostics Market Insight
The Indonesia malaria diagnostics market is growing steadily, fueled by endemic malaria in rural and remote regions. RDTs provide fast, affordable, and portable testing for community health programs, while molecular diagnostics are used in hospitals for accurate case confirmation. Government-led malaria control campaigns and NGO-supported awareness programs are expanding coverage. Mobile testing initiatives improve access in underserved regions. Integration with digital health platforms enhances reporting and surveillance. The combined focus on accessibility, affordability, and accuracy is driving market expansion.
Europe Malaria Diagnostics Market Insight
The Europe malaria diagnostics market is projected to expand at a substantial CAGR during the forecast period, primarily driven by the need for accurate testing of imported malaria cases and surveillance of travelers returning from endemic regions. Hospitals, diagnostic laboratories, and clinics are increasingly relying on molecular diagnostics and RDTs for rapid, reliable results. European healthcare providers value diagnostics that enable precise treatment planning and integration with national disease monitoring systems. The region’s emphasis on research, advanced laboratory infrastructure, and skilled healthcare personnel further supports adoption. Public health initiatives and awareness campaigns enhance the accessibility and use of malaria diagnostics across Europe.
United Kingdom Malaria Diagnostics Market Insight
The U.K. malaria diagnostics market is projected to grow at a substantial CAGR during the forecast period, driven by the need for accurate testing of imported malaria cases and travelers returning from endemic regions. Hospitals, travel clinics, and diagnostic laboratories rely on molecular diagnostics and RDTs for rapid and precise results. Public health initiatives and national surveillance programs enhance disease monitoring. Healthcare providers prioritize accurate treatment planning and integration with electronic health records. Increasing awareness among travelers and clinicians supports adoption. The combination of advanced infrastructure, skilled workforce, and reliable diagnostic solutions drives market expansion.
Germany Malaria Diagnostics Market Insight
The Germany malaria diagnostics market is expected to expand at a considerable CAGR during the forecast period, fueled by the rising number of imported malaria cases and demand for precise species identification. Hospitals and diagnostic laboratories are adopting molecular diagnostic platforms alongside RDTs for accurate and timely diagnosis. National surveillance programs and public health initiatives strengthen disease monitoring and outbreak management. Integration with digital health systems enhances reporting and treatment coordination. Growing awareness among clinicians and travelers further supports adoption. High-quality healthcare infrastructure and focus on accuracy continue to drive market growth.
Malaria Diagnostics Market Share
The Malaria Diagnostics industry is primarily led by well-established companies, including:
- Abbott (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Siemens Healthcare AG (Germany)
- Access Bio, Inc. (South Korea)
- SD Biosensor, Inc. (South Korea)
- Premier Medical Corporation Pvt. Ltd. (India)
- Meridian Bioscience, Inc. (U.S.)
- BD (U.S.)
- F. Hoffmann La Roche Ltd. (Switzerland)
- QIAGEN (Netherlands)
- Sysmex Corporation (Japan)
- Olympus Corporation (Japan)
- Wako Chemicals, Inc. (Japan)
- Novartis AG (Switzerland)
- Leica Microsystems GmbH (Germany)
- ZeptoMetrix Corporation (U.S.)
- Omega Diagnostics Group PLC (U.K.)
- QuidelOrtho Corporation (U.S.)
- Trinity Biotech plc (Ireland)
What are the Recent Developments in Global Malaria Diagnostics Market?
- In October 2025, a novel portable diagnostics platform named Dragonfly malaria test demonstrated the ability to detect asymptomatic and low‑density malaria infections (as low as 0.6 parasites/µL) from a finger‑prick sample in under 45 minutes. This is a major breakthrough, because asymptomatic carriers often escape detection but continue to fuel transmission; by enabling fast, sensitive detection outside traditional labs, Dragonfly could be a game‑changer in malaria elimination efforts
- In January 2025, World Health Organization (WHO) pre‑qualified the first diagnostic test for Glucose-6-phosphate dehydrogenase deficiency (G6PD) a critical step to ensure safe administration of radical‑cure treatments for Plasmodium vivax malaria. The prequalification means healthcare programs can now more confidently screen for G6PD deficiency before giving anti‑relapse drugs, reducing the risk of drug‑induced haemolysis and enabling safer, wider use of effective therapies for P. vivax malaria worldwide
- In April 2024, researchers at Rice University unveiled a new point‑of‑care malaria test a microfluidic immunoassay capable of detecting the malaria biomarker PfHRP2 in whole blood in just 15 minutes, making it significantly faster (≈12×) than traditional lab‑based tests; this test is designed to be field‑deployable and user‑friendly, offering potential for rapid, accurate diagnosis even in low‑resource or rural settings
- In March 2024, Roche received regulatory clearance from the U.S. Food and Drug Administration (FDA) for its cobas Malaria test the first molecular test approved to screen blood and organ donors for malaria. This approval provides a high‑sensitivity, DNA/RNA‑based screening method for the five major human malaria parasites, significantly improving the safety of the global blood supply by enabling detection of infections that traditional methods may miss
- In March 2024, along with the regulatory approval, researchers announced a new point‑of‑care (POC) malaria diagnostic that is 12 times faster than many conventional laboratory tests combining speed with molecular-level sensitivity. This development promises to bring high‑accuracy diagnostics to resource-limited settings, helping overcome key limitations of existing methods and enabling broader use in endemic regions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.