Global Microbiome Based Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
3.17 Billion
USD
18.29 Billion
2024
2032
USD
3.17 Billion
USD
18.29 Billion
2024
2032
| 2025 –2032 | |
| USD 3.17 Billion | |
| USD 18.29 Billion | |
|
|
|
|
Microbiome-Based Therapeutics Market Size
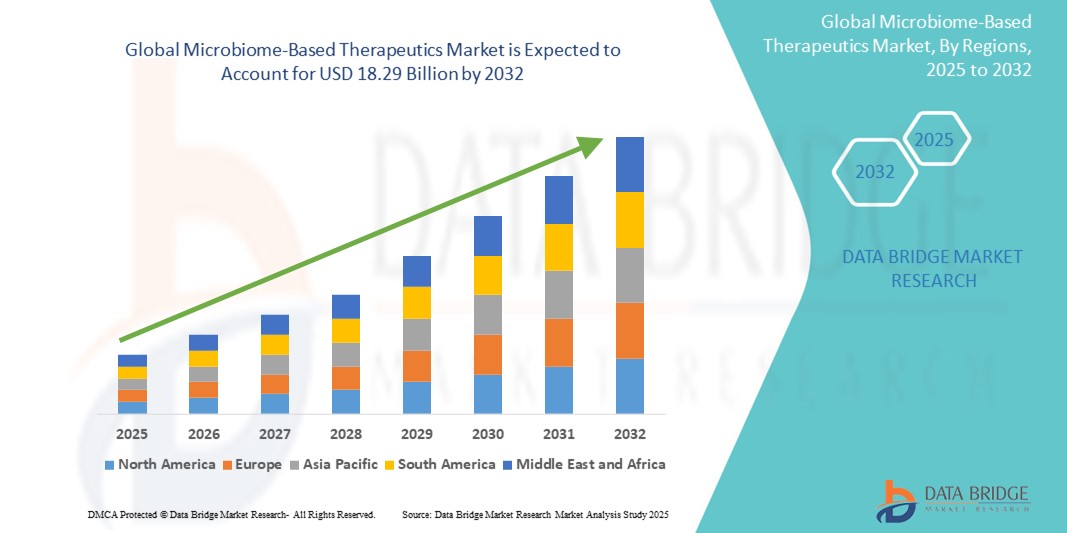
- The global Microbiome-Based Therapeutics market size was valued at USD 3.17 billion in 2024 and is expected to reach USD 18.29 billion by 2032, at a CAGR of 24.50% during the forecast period
- The market's growth is largely fueled by the growing understanding of the human microbiome's role in health and disease, driving new therapeutic avenues. The rising prevalence of chronic diseases linked to microbiome imbalances, such as IBD and CDI, is boosting demand for effective treatments
- Furthermore, advances in genomic technologies and AI-driven analytics are enhancing personalized therapies. Significant investments in R&D, supportive regulations, and increasing collaborations are accelerating the commercialization of these novel solutions, significantly boosting industry growth
Microbiome-Based Therapeutics Market Analysis
- Microbiome-based therapeutics, focusing on modulating the human microbiome to treat or prevent diseases, are becoming increasingly vital in modern healthcare due to a deeper understanding of the microbiome's role in health and disease, alongside advancements in biotechnology
- The escalating demand for these therapeutics is primarily fueled by the rising prevalence of chronic diseases linked to microbiome dysbiosis, such as inflammatory bowel disease (IBD) and Clostridium difficile infection (CDI), and the growing need for novel and effective treatment options beyond traditional therapies
- North America dominates the microbiome-based therapeutics market with the largest revenue share of 76.3% in 2025, characterized by established research infrastructure, significant R&D investments, and a strong presence of key industry players, particularly in the U.S., which benefits from a robust biotechnology sector and supportive regulatory environment driving rapid development and commercialization
- Asia-Pacific is expected to be the fastest growing region in the microbiome-based therapeutics market with a CAGR of 24.5%, during the forecast period due to increasing healthcare spending, rising awareness about gut health, growing research activities, and government support for personalized medicine in countries
- Fecal microbiota therapy segment is expected to dominate the microbiome-based therapeutics market with a market share of 92.1% in 2025, driven by its established efficacy, particularly in treating recurrent Clostridium difficile infection (CDI), and its increasing application in other gastrointestinal disorders
Report Scope and Microbiome-Based Therapeutics Market Segmentation
|
Attributes |
Microbiome-Based Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Microbiome-Based Therapeutics Market Trends
“Advancements in Precision Medicine and AI-Driven Therapeutic Development”
- A significant and accelerating trend in the global microbiome-based therapeutics market is the integration of advanced technologies such as AI, machine learning, and high-throughput sequencing to enable the development of highly personalized and precise treatments. This fusion of technologies is fundamentally reshaping how microbiome-related diseases are diagnosed and treated
- For instance, companies are leveraging AI and bioinformatics to analyze complex microbiome data, identify specific microbial signatures associated with diseases, and predict patient responses to therapies. This allows for the development of targeted interventions, moving beyond broad-spectrum approaches. Similarly, advances in sequencing technologies enable in-depth profiling of individual microbiomes, providing the basis for personalized therapeutic strategies
- AI integration in microbiome therapeutics enables features such as identifying novel beneficial strains, optimizing microbial consortia for specific conditions, and predicting potential drug-microbiome interactions. For instance, AI algorithms can learn complex relationships between gut microbiota composition and disease progression, leading to more intelligent therapeutic design. Furthermore, advanced analytics are crucial for deciphering the functional aspects of microbial communities and their metabolites, opening doors for novel drug candidates
- This trend towards more intelligent, precise, and personalized microbiome interventions is fundamentally reshaping therapeutic development. Consequently, companies are investing heavily in AI platforms and genomic sequencing capabilities to accelerate drug discovery and development in this rapidly evolving field
- The demand for microbiome-based therapeutics that offer precision and personalized approaches is growing rapidly across both patient and clinical sectors, as healthcare increasingly prioritizes targeted and effective solutions for complex diseases
Microbiome-Based Therapeutics Market Dynamics
Driver
“Rising Prevalence of Chronic Diseases and Advancements in Microbiome Research”
- The increasing global burden of chronic diseases, particularly gastrointestinal disorders, metabolic syndromes, and certain autoimmune conditions, coupled with rapid advancements in understanding the human microbiome, is a significant driver for the heightened demand for microbiome-based therapeutics
- For instance, the high recurrence rate of Clostridium difficile infection (CDI) with traditional antibiotics has created a strong need for effective alternatives such as Fecal Microbiota Transplantation (FMT), which has shown remarkable efficacy. Similarly, increasing cases of inflammatory bowel disease (IBD) and metabolic disorders are prompting deeper exploration into microbiome-targeted treatments
- As scientists and clinicians gain a more profound understanding of the intricate links between microbiome dysbiosis and the pathogenesis of various chronic conditions, the potential for novel therapeutic interventions expands dramatically. This includes identifying specific microbial imbalances that contribute to disease and developing strategies to restore a healthy microbiome
- Furthermore, significant advancements in research technologies, such as next-generation sequencing and bioinformatics, are enabling detailed analysis of microbial communities. This is accelerating the discovery of new therapeutic targets and the development of innovative approaches such as live biotherapeutic products (LBPs), microbial consortia, and even genetically engineered microbes
- The growing recognition of the microbiome's role in overall health and disease, coupled with the limitations and side effects of conventional treatments for many chronic conditions, is compelling both researchers and pharmaceutical companies to invest heavily in this promising field. This creates a powerful impetus for the adoption and development of microbiome-based therapies across various disease areas
Restraint/Challenge
“Complex Regulatory Pathways and Manufacturing Hurdles for Live Biotherapeutics”
- The intricate and evolving regulatory landscape, coupled with significant manufacturing complexities, poses a substantial challenge to the broader commercialization and market penetration of microbiome-based therapeutics, especially Live Biotherapeutic Products (LBPs). As these products involve living organisms, they do not fit neatly into traditional pharmaceutical frameworks, creating uncertainties for developers
- For instance, the classification of microbiome products (as drugs, biologics, or even food/supplements) can vary across different regulatory bodies globally, leading to confusion and extended development timelines. High-profile reports of clinical trial failures or unexpected safety signals, though rare, can also increase regulatory scrutiny and investor caution
- Addressing these regulatory ambiguities requires close collaboration between companies and regulatory agencies to establish clear guidelines for preclinical studies, clinical trial design, and approval pathways. Companies such as Seres Therapeutics and Finch Therapeutics have navigated these complex pathways, highlighting the rigor required. In addition, the inherent biological complexity of live microbial products makes manufacturing particularly challenging
- Ensuring consistency, viability, purity, and potency of live organisms throughout the manufacturing, storage, and distribution process demands specialized infrastructure (for instance, anaerobic conditions), stringent quality control, and significant investment. This contrasts sharply with the more standardized production of small molecule drugs, contributing to higher production costs and scalability issues
- While scientific understanding is rapidly advancing, the need for robust evidence demonstrating long-term safety and efficacy, coupled with the high costs associated with specialized R&D and manufacturing for live biotherapeutics, can hinder widespread adoption and limit accessibility, particularly in price-sensitive markets
- Overcoming these challenges through global regulatory harmonization, investment in advanced manufacturing technologies, and increased standardization will be vital for sustained market growth
Microbiome-Based Therapeutics Market Scope
The market is segmented on the basis of type and application.
- By Type
On the basis of type, the microbiome-based therapeutics market is segmented into fecal microbiota therapy (FMT) and microbiome drugs. The fecal microbiota therapy (FMT) segment currently holds the largest market revenue share, accounting for 92.1%. This dominance is due to its proven high efficacy in treating recurrent Clostridium difficile infection (CDI), with success rates often over 80%. Its established effectiveness and increasing regulatory approvals, such as those from the FDA, drive its significant market presence. Ongoing research for other gut issues further supports this segment.
The microbiome drugs segment is anticipated to witness the fastest growth rate, with some reports projecting a CAGR of 49.1% from 2025 to 2032. This growth is fueled by substantial R&D investment and a rich pipeline of novel drug candidates. These designed microbial products promise more targeted and standardized treatments across a wider range of diseases, with many still in development, driving this segment's rapid expansion.
- By Application Type
On the basis of application type, the microbiome-based therapeutics market is segmented into C. difficile Infection (CDI), Crohn’s disease, inflammatory bowel disease (IBD), diabetes, and others. The C. difficile Infection (CDI) application held the largest market revenue share, in 2024. This is primarily due to the significant unmet medical need for recurrent CDI, where microbiome therapies excel. This segment is projected for strong growth, with a CAGR of about 33.8% from 2024 to 2032
The Crohn's disease segment holds a significant share and is expected to see fast growth due to the increasing understanding of the gut microbiome's role and limitations of current treatments. The broader Inflammatory Bowel Disease (IBD) segment also held a substantial share, driven by rising cases and deepening scientific insights. The Diabetes segment is an emerging and fast-growing area, as evidence links gut microbiome imbalances to diabetes development.
Microbiome-Based Therapeutics Market Regional Analysis
- North America dominates the microbiome-based therapeutics market with the largest revenue share of 76.3% in 2024, driven by established research infrastructure, significant R&D investments, and a strong presence of key industry players, particularly in the U.S., which benefits from a robust biotechnology sector and supportive regulatory environment driving rapid development and commercialization
- Consumers and healthcare providers in the region highly value innovative treatment options for chronic diseases such as IBD and diabetes, which are prevalent.
- This widespread adoption is further supported by high healthcare spending, a technologically advanced population, and numerous collaborations between academic institutions and pharmaceutical companies, establishing North America as a favored hub for microbiome therapeutic development and adoption.
U.S. Microbiome-Based Therapeutics Market Insight
The U.S. microbiome-based therapeutics market captured a significant revenue share of 70.5% within North America, fueled by robust R&D infrastructure and substantial private and public investments in biotechnology. Consumers and healthcare providers are increasingly prioritizing novel treatments for chronic diseases linked to the microbiome, such as IBD and diabetes. The growing recognition of gut health's role in overall well-being, combined with strong regulatory support and numerous collaborations between academia and biopharma companies, further propels the market. Moreover, the increasing adoption of personalized medicine approaches, supported by advanced sequencing technologies, is significantly contributing to the market's expansion
Europe Microbiome-Based Therapeutics Market Insight
The European microbiome-based therapeutics market is projected to expand at a substantial CAGR of 35.9%. This growth is primarily driven by increasing awareness of the gut microbiome's role in health and disease, coupled with rising research efforts across the continent. The region's well-established healthcare systems and evolving regulatory frameworks provide a conducive environment for developing and commercializing microbiome-based therapies. European consumers are also drawn to innovative solutions for chronic conditions. The region is experiencing significant growth across various therapeutic applications, with research and development being incorporated into both established pharmaceutical companies and emerging biotech firms.
U.K. Microbiome-Based Therapeutics Market Insight
The U.K. microbiome-based therapeutics market is anticipated to grow at a noteworthy CAGR of 37.1%. This growth is driven by increasing research and development activities and a rising focus on novel treatment modalities for conditions such as C. difficile infection and inflammatory bowel diseases. In addition, increasing awareness of gut health among both healthcare professionals and the public is encouraging the adoption of microbiome-based solutions. The UK’s robust scientific infrastructure and strategic investments in biotech, alongside its active role in global clinical trials, are expected to continue to stimulate market growth.
Germany Microbiome-Based Therapeutics Market Insight
The German microbiome-based therapeutics market is expected to expand at a considerable CAGR during the forecast period. This growth is fueled by increasing awareness of the human microbiome's impact on health and a strong demand for innovative, evidence-based solutions. Germany’s well-developed healthcare system and supportive regulatory environment, combined with its emphasis on cutting-edge medical research, promote the adoption of microbiome therapies, particularly in treating gastrointestinal and metabolic disorders. The integration of microbiome research with personalized medicine approaches is also becoming increasingly prevalent, aligning with local consumer expectations for precise and effective treatments
Asia-Pacific Microbiome-Based Therapeutics Market Insight
The Asia-Pacific microbiome-based therapeutics market is poised for the fastest growth, with some reports suggesting a CAGR of 24.5%. This surge is driven by increasing urbanization, rising disposable incomes, and significant technological advancements in countries such as China, Japan, and India. The region's growing inclination towards advanced healthcare solutions, supported by government initiatives promoting biotechnology and personalized medicine, is accelerating the adoption of microbiome therapies. Furthermore, as APAC emerges as a hub for scientific research and manufacturing, the accessibility and affordability of these therapies are expanding to a wider patient base
Japan Microbiome-Based Therapeutics Market Insight
The Japan microbiome-based therapeutics market is gaining momentum, projected to grow at a CAGR of 22.4 %. This is driven by the country’s high-tech healthcare culture, an aging population, and a strong demand for novel therapeutic solutions, particularly for chronic diseases. The Japanese market places significant emphasis on scientific innovation, and the adoption of microbiome therapies is fueled by increasing research into gut-brain axis disorders and metabolic conditions. The integration of microbiome research with other advanced medical technologies is also fueling growth.
India Microbiome-Based Therapeutics Market Insight
The India microbiome-based therapeutics market is experiencing significant growth, although its specific share within Asia Pacific is comparatively nascent. It is expected to grow at a CAGR of 21.7%, attributed to the country's expanding middle class, rapid urbanization, and rising awareness of gut health. India faces a growing burden of chronic diseases, driving demand for novel treatments. The push towards advanced healthcare infrastructure and the availability of increasing domestic research initiatives are key factors propelling the market in India, alongside a growing interest in probiotics and functional foods as precursors to more complex microbiome therapies
Microbiome-Based Therapeutics Market Share
The microbiome-based therapeutics industry is primarily led by well-established companies, including:
- Seres Therapeutics (U.S.)
- Ferring B.V. (Switzerland)
- Vedanta Biosciences, Inc. (U.S.)
- BiomX (Israel)
- MaaT Pharma (France)
- Enterome SA (France)
- Microbiotica (U.K.)
- Pendulum. (U.S.)
- Axial Therapeutics, Inc. (U.S.)
- Aimmune Therapeutics, Inc. (U.S.)
- Gilead Sciences, Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Johnson & Johnson Services, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- F. Hoffmann-La Roche (Switzerland)
- Sanofi (France)
- AbbVie Inc. (U.S.)
- Bristol Myers Squibb Company (U.S.)
- Amgen Inc. (U.S.)
Latest Developments in Global Microbiome-Based Therapeutics Market
- In April 2025, the UC San Diego Center for Microbiome Innovation announced Haleon as a new industry partner. This collaboration aims to unite CMI's microbiome expertise with Haleon's science and clinical capabilities to accelerate microbiome discoveries through joint research programs, especially exploring solutions for consumer health areas such as healthy aging, cardiovascular health, and general well-being
- In May 2024, Vedanta Biosciences announced the enrollment of the first patient in its pivotal Phase 3 RESTORATiVE303 study of VE303, an orally administered defined bacterial consortium candidate for the prevention of recurrent C. difficile infection. This significant step highlights the ongoing progression of defined consortium approaches, moving beyond donor-derived therapies
- In January 2024, Vedanta Biosciences Inc. announced a significant collaboration with an academic institution to delve deeper into the gut microbiome’s intricate role in metabolic health and explore its potential for therapeutic applications. This partnership aims to leverage cutting-edge research to develop novel approaches for metabolic disorders
- In April 2023, Ferring Pharmaceuticals received FDA approval for REBYOTA (fecal microbiota, live – jslm), a microbiota-based live therapeutic indicated for the prevention of recurrence of Clostridioides difficile infection (CDI) in individuals 18 years of age and older, following antibiotic treatment for recurrent CDI. This approval was a key milestone, providing a ready-to-use formulation
- In March 2023, Seres Therapeutics received FDA approval for Vowst (formerly SER-109), the first orally administered microbiota-based therapeutic for the prevention of recurrent Clostridioides difficile infection (CDI) in adults. This landmark approval marked a significant advancement in microbiome medicine, offering a new treatment option for a challenging condition
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.