Global Microbiome Modulator Market
Market Size in USD Billion
CAGR :
% 
 USD
3.16 Billion
USD
8.57 Billion
2024
2032
USD
3.16 Billion
USD
8.57 Billion
2024
2032
| 2025 –2032 | |
| USD 3.16 Billion | |
| USD 8.57 Billion | |
|
|
|
|
Microbiome Modulator Market Analysis
Microbiomes refer to a group of microorganisms that live on the skin layer, in saliva and the gastrointestinal tract (GIT). Bacteria, archaea and fungi all belong to the category of microbiomes. The microbiomes are helpful in performing certain task that are overall beneficial for human health.
Microbiome Modulator Market Size
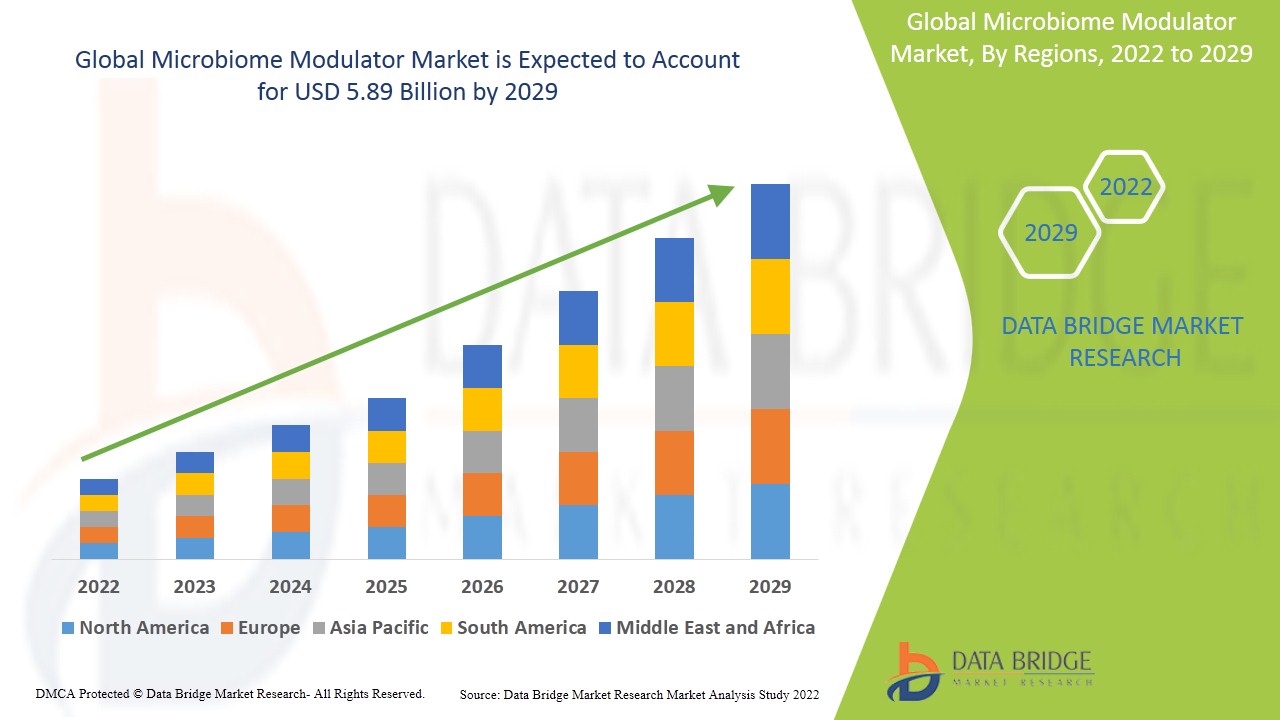
Global microbiome modulator market size was valued at USD 3.16 billion in 2024 and is projected to reach USD 8.57 billion by 2032, with a CAGR of 13.30% during the forecast period of 2025 to 2032.
Report Scope and Market Segmentation
|
Attributes |
Microbiome Modulator Key Market Insights |
|
Segmentation |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Key Market Players |
Sanofi, Procter & Gamble, Bayer AG, DSM, DuPont., Yakult Honsha Co., Ltd., BioGaia AB, Probi AB, Chr. Hansen Holding A/S, Ingredion Incorporated, Immuron Ltd, Pharmavite, MICROBIOME THERAPEUTICS, LLC, Otsuka America Pharmaceutical, Inc., Quorum Innovations, Merck Sharp & Dohme Corp., Symbiotix Biotherapies, Inc., and Ritter Pharmaceuticals |
Microbiome Modulator Market Dynamics
Drivers
- Lifestyle disorders
Surging incidence rate of chronic health and lifestyle disorders in patients is directly propelling the demand for effective medical treatment. This indicates that the increasing prevalence of hypertension, heart attack, cancer, Alzheimer's disease, asthma, chronic liver disease, diabetes, and osteoporosis will increase the demand for microbiome modulators.
- Increasing investment for healthcare infrastructure
Another significant factor influencing the market's growth rate is the rising healthcare expenditure, which helps in improving its infrastructure. Growth and expansion of healthcare industry especially in the developing economies would invite the use and application of new and advanced medial technologies, equipment and drugs. This will directly increase the demand for microbiome modulator treatment.
- Research and development activities
The growing number of strategic collaboration between public and private market players is inducing growth in the number of research and development activities on a daily basis. These research and development proficiencies are being conducted in novel drugs and medical technologies that will propel the demand for microbiome modulator treatment.
Furthermore, rising initiatives by public and private organizations to spread awareness increasing number of geriatric population and increased demand for prevention medicines with minimum side effects are the factors that will expand the market growth rate. Other factors such as increase in the demand for effective therapies, availability of cost effective drugs and rising adoption rate for early diagnostic procedures will positively impact the market's growth rate.
Opportunities
Rise in the level of personal disposable income and growing awareness in the developing economies pertaining to the availability of treatment options will further create lucrative market growth opportunities. Rising medical tourism globally, growing number of collaborations between market players, increasing involvement of key players in product development and high potential of growth in the untapped market will create enough market growth opportunities.
Restraints/Challenges
However, high costs associated with research and development proficiencies and lack of effective treatment which cut down the scope of innovative medication will emerge as one of the biggest restraints for the market. Dearth of awareness and required infrastructural facilities in the underdeveloped and backward economies, stringent government regulations towards approval of biosimilars and unfavourable conditions arising due to COVID-19 outbreak will further derail the market growth rate. Slow rate of approval for drugs and inhibitors, unmet medical needs and increasing cases of patent expiry will also challenge the market growth rate.
This microbiome modulator market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on microbiome modulator market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Microbiome Modulator Market Scope
The microbiome modulator market is segmented on the basis of products, application, end-users and distribution channel. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Products
- Prebiotics
- Creams
- Probiotics
- Drugs
- Others
Application
- Digestive Health
- Immune Health
- Oral Health
- Others
End-Users
- Hospitals
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Others
Microbiome Modulator Market Regional Analysis
The microbiome modulator market is analysed and market size insights and trends are provided by country, products, application, end-users and distribution channel as referenced above.
The countries covered in the microbiome modulator market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the microbiome modulator market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the presence of major key players, well-developed healthcare infrastructure in this region and increase in the incidence of proctitis and favorable reimbursement policies. Asia-Pacific on the other hand is projected to exhibit the highest growth rate during the forecast period due to the increase in the awareness about proctitis in developing countries and growing government support.
The country section of the report also provides individual market impacting factors and changes in regulations in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, disease epidemiology and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Microbiome Modulator Market Share
The microbiome modulator market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to microbiome modulator market.
Microbiome Modulator Market Leaders Operating in the Market Are:
- Sanofi, Procter & Gamble
- Bayer AG
- DSM
- DuPont.
- Yakult Honsha Co., Ltd.
- BioGaia AB
- Probi AB, Chr
- Hansen Holding A/S
- Ingredion Incorporated
- Immuron Ltd
- Pharmavite
- MICROBIOME THERAPEUTICS, LLC
- Otsuka America Pharmaceutical, Inc.
- Quorum Innovations
- Merck Sharp & Dohme Corp.
- Symbiotix Biotherapies, Inc.
- Ritter Pharmaceuticals
Latest Developments in Microbiome Modulator Market
- In 2020, Vedanta Biosciences (US) received funding from the Biomedical Advanced Research and Development Authority (BARDA) of USD 7.4 million to advance the clinical development of VE303 for high-risk Clostridioides difficile infection (CDI)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL MICROBIOME MODULATOR MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL MICROBIOME MODULATOR MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 MARKET GUIDE
2.2.4 COMPANY POSITIONING GRID
2.2.5 COMPANY MARKET SHARE ANALYSIS
2.2.6 MULTIVARIATE MODELLING
2.2.7 TOP TO BOTTOM ANALYSIS
2.2.8 STANDARDS OF MEASUREMENT
2.2.9 VENDOR SHARE ANALYSIS
2.2.10 SALES VOLUME
2.2.11 EPIDEMIOLOGY MODELLING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL MICROBIOME MODULATOR MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR MICROBIOME MODULATOR MARKET
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE MICROBIOME MODULATOR MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE MICROBIOME MODULATOR MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE MICROBIOME MODULATOR MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR MICROBIOME MODULATOR MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
10.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.13.1 FORECAST MARKET OUTLOOK
10.13.2 CROSS COMPETITION
10.13.3 THERAPEUTIC PORTFOLIO
10.13.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 MARKET OVERVIEW
13.1 DRIVERS
13.2 RESTRAINTS
13.3 OPPORTUNITIES
13.4 CHALLENGES
14 GLOBAL MICROBIOME MODULATOR MARKET, BY PRODUCT TYPE
14.1 OVERVIEW
14.2 PREBIOTICS
14.2.1 BY TYPE
14.2.1.1. MANNAN-OLIGOSACCHARIDES
14.2.1.2. GALACTOOLIGOSACCHARIDES
14.2.1.3. FRUCTO-OLIGOSACCHARIDE
14.2.1.4. POLYDEXTROSE
14.2.1.5. OTHERS
14.2.2 BY CATEGORY
14.2.2.1. CONVENTIONAL
14.2.2.2. ORGANIC
14.2.3 BY SOURCE
14.2.3.1. PLANT-BASED
14.2.3.2. ANIMAL BASED
14.2.3.3. OTHERS (IF ANY)
14.2.4 BY PRODUCT TYPE
14.2.4.1. ORIGINAL
14.2.4.2. BLENDED
14.2.5 OTHERS
14.3 PROBIOTICS
14.3.1 BY TYPE
14.3.1.1. PROBIOTIC DIETARY SUPPLEMENTS
14.3.1.2. PROBIOTIC DAIRY YOGHURT
14.3.1.3. YOGHURT DRINKS
14.3.1.4. PROBIOTIC NON-DAIRY FOOD AND BEVERAGE
14.3.2 BY CATEGORY
14.3.2.1. CONVENTIONAL
14.3.2.2. ORGANIC
14.3.3 BY SOURCE
14.3.3.1. PLANT-BASED
14.3.3.2. ANIMAL BASED
14.3.3.3. OTHERS (IF ANY)
14.3.4 BY PRODUCT TYPE
14.3.4.1. ORIGINAL
14.3.4.2. BLENDED
14.3.5 OTHERS
14.4 SYNBIOTICS
14.4.1 BY TYPE
14.4.1.1. SYNBIOTIC SUPPLEMENTS
14.4.1.2. SYNBIOTIC FUNCTIONAL FOODS AND BEVERAGES
14.4.1.3. SYNBIOTIC FOODS FOR SPECIFIC CONDITIONS
14.4.1.4. OTHERS
14.4.2 BY CATEGORY
14.4.2.1. CONVENTIONAL
14.4.2.2. ORGANIC
14.4.3 BY SOURCE
14.4.3.1. PLANT-BASED
14.4.3.2. ANIMAL BASED
14.4.3.3. OTHERS (IF ANY)
14.4.4 BY PRODUCT TYPE
14.4.4.1. ORIGINAL
14.4.4.2. BLENDED
14.4.5 OTHERS
14.5 METAPROBIOTICS
14.5.1 BY TYPE
14.5.1.1. SUPPLEMENTS
14.5.1.2. FUNCTIONAL FOODS
14.5.1.3. BEVERAGES
14.5.1.4. OTHERS
14.5.2 BY CATEGORY
14.5.2.1. CONVENTIONAL
14.5.2.2. ORGANIC
14.5.3 BY SOURCE
14.5.3.1. PLANT-BASED
14.5.3.2. ANIMAL BASED
14.5.3.3. OTHERS (IF ANY)
14.5.4 BY PRODUCT TYPE
14.5.4.1. ORIGINAL
14.5.4.2. BLENDED
14.5.5 OTHERS
14.6 DRUGS/THERAPIES
14.6.1 FECAL MICROBIOTA TRANSPLANTATION (FMT)
14.6.1.1. REBYOTA
14.6.1.2. VOWST
14.6.1.3. FINCH THERAPEUTICS' FIN-524
14.6.1.4. OTHERS
14.6.2 PROBIOTIC DRUGS
14.6.2.1. VSL 3
14.6.2.2. FLORASTOR
14.6.2.3. ALIGN
14.6.2.4. OTHERS
14.6.3 ANTIBIOTIC-BASED MICROBIOME MODULATORS
14.6.3.1. INTESTINAL MICROFLORA MODULATOR (RIFAXIMIN):
14.6.3.2. XIFAXAN
14.6.3.3. OTHERS
14.6.4 OTHERS
14.7 OTHERS
14.7.1 PHYTOBIOTICS
14.7.2 POSTBIOTICS
14.7.3 OTHER MODULATORS
15 GLOBAL MICROBIOME MODULATOR MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 ORAL
15.2.1 TABLETS
15.2.2 CAPSULES
15.2.3 POWDER
15.2.4 SOLUTIONS
15.2.5 OTHERS
15.3 PARENTERAL
15.3.1 INTRAVENEOUS
15.3.2 SUBCUTANEOUS
15.3.3 OTHERS
15.4 TOPICAL
15.5 OTHERS
16 GLOBAL MICROBIOME MODULATOR MARKET, BY POPULATION TYPE
16.1 MALE
16.1.1 PEDIATRIC
16.1.2 ADULT
16.1.3 GERIATRIC
16.2 FEMALE
16.2.1 PEDIATRIC
16.2.2 ADULT
16.2.3 GERIATRIC
17 GLOBAL MICROBIOME MODULATOR MARKET, BY APPLICATION
17.1 OVERVIEW
17.2 ORAL HEALTH
17.3 SKIN HEALTH
17.4 IMMUNE HEALTH
17.5 DIGESTIVE HEALTH
17.6 METABOLIC HEALTH
17.7 OTHERS
18 GLOBAL MICROBIOME MODULATOR MARKET, BY END USER
18.1 OVERVIEW
18.2 HOSPITALS
18.2.1 BY TYPE
18.2.1.1. PUBLIC
18.2.1.2. PRIVATE
18.2.2 BY LEVEL
18.2.2.1. TIER 1
18.2.2.2. TIER 2
18.2.2.3. TIER 3
18.3 SPECIALTY CLINICS
18.3.1 PUBLIC
18.3.2 PRIVATE
18.4 HOME HEALTHCARE
18.5 ACADEMIC AND RESEARCH INSTITUTES
18.6 OTHERS
19 GLOBAL MICROBIOME MODULATOR MARKET, BY DISTRIBUTION CHANNEL
19.1 OVERVIEW
19.2 DIRECT TENDER
19.3 RETAIL SALES
19.3.1 OFFLINES SALES
19.3.1.1. HOSPITAL PHARMACIES
19.3.1.2. RETAIL PHARMACIES
19.3.1.3. OTHERS
19.3.2 ONLINE SALES
19.3.2.1. E-STORES
19.3.2.2. COMPANY WEBSITE
19.3.2.3. OTHERS
19.4 OTHERS
20 GLOBAL MICROBIOME MODULATOR MARKET, BY GEOGRAPHY
GLOBAL MICROBIOME MODULATOR MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS
REPRESENTED IN THIS CHAPTER BY COUNTRY)
20.1 NORTH AMERICA
20.1.1 U.S.
20.1.2 CANADA
20.1.3 MEXICO
20.2 EUROPE
20.2.1 GERMANY
20.2.2 FRANCE
20.2.3 U.K.
20.2.4 HUNGARY
20.2.5 LITHUANIA
20.2.6 AUSTRIA
20.2.7 IRELAND
20.2.8 NORWAY
20.2.9 POLAND
20.2.10 ITALY
20.2.11 SPAIN
20.2.12 RUSSIA
20.2.13 TURKEY
20.2.14 NETHERLANDS
20.2.15 SWITZERLAND
20.2.16 REST OF EUROPE
20.3 ASIA-PACIFIC
20.3.1 JAPAN
20.3.2 CHINA
20.3.3 TAIWAN
20.3.4 SOUTH KOREA
20.3.5 INDIA
20.3.6 AUSTRALIA
20.3.7 SINGAPORE
20.3.8 THAILAND
20.3.9 MALAYSIA
20.3.10 INDONESIA
20.3.11 PHILIPPINES
20.3.12 VIETNAM
20.3.13 REST OF ASIA-PACIFIC
20.4 SOUTH AMERICA
20.4.1 BRAZIL
20.4.2 ECUADOR
20.4.3 CHILE
20.4.4 COLOMBIA
20.4.5 VENEZUELA
20.4.6 ARGENTINA
20.4.7 PERU
20.4.8 CURAÇAO
20.4.9 PARAGUAY
20.4.10 URUGUAY
20.4.11 TRINIDAD AND TOBAGO
20.4.12 REST OF SOUTH AMERICA
20.5 MIDDLE EAST AND AFRICA
20.5.1 SOUTH AFRICA
20.5.2 SAUDI ARABIA
20.5.3 UAE
20.5.4 EGYPT
20.5.5 KUWAIT
20.5.6 ISRAEL
20.5.7 BOLIVIA
20.5.8 REST OF MIDDLE EAST AND AFRICA
20.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
21 GLOBAL MICROBIOME MODULATOR MARKET, SWOT AND DBMR ANALYSIS
22 GLOBAL MICROBIOME MODULATOR MARKET, COMPANY LANDSCAPE
22.1 COMPANY SHARE ANALYSIS: GLOBAL
22.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
22.3 COMPANY SHARE ANALYSIS: EUROPE
22.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
22.5 MERGERS & ACQUISITIONS
22.6 NEW PRODUCT DEVELOPMENT & APPROVALS
22.7 EXPANSIONS
22.8 REGULATORY CHANGES
22.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
23 GLOBAL MICROBIOME MODULATOR MARKET, COMPANY PROFILE
23.1 PROBI AB
23.1.1 COMPANY OVERVIEW
23.1.2 REVENUE ANALYSIS
23.1.3 GEOGRAPHIC PRESENCE
23.1.4 PRODUCT PORTFOLIO
23.1.5 RECENT DEVELOPMENTS
23.2 IMMURON LTD.
23.2.1 COMPANY OVERVIEW
23.2.2 REVENUE ANALYSIS
23.2.3 GEOGRAPHIC PRESENCE
23.2.4 PRODUCT PORTFOLIO
23.2.5 RECENT DEVELOPMENTS
23.3 BIOGAIA
23.3.1 COMPANY OVERVIEW
23.3.2 REVENUE ANALYSIS
23.3.3 GEOGRAPHIC PRESENCE
23.3.4 PRODUCT PORTFOLIO
23.3.5 RECENT DEVELOPMENTS
23.4 BAYERS AG
23.4.1 COMPANY OVERVIEW
23.4.2 REVENUE ANALYSIS
23.4.3 GEOGRAPHIC PRESENCE
23.4.4 PRODUCT PORTFOLIO
23.4.5 RECENT DEVELOPMENTS
23.5 POSTBIOTIC
23.5.1 COMPANY OVERVIEW
23.5.2 REVENUE ANALYSIS
23.5.3 GEOGRAPHIC PRESENCE
23.5.4 PRODUCT PORTFOLIO
23.5.5 RECENT DEVELOPMENTS
23.6 SANOFI
23.6.1 COMPANY OVERVIEW
23.6.2 REVENUE ANALYSIS
23.6.3 GEOGRAPHIC PRESENCE
23.6.4 PRODUCT PORTFOLIO
23.6.5 RECENT DEVELOPMENTS
23.7 PROCTER & GAMBLE
23.7.1 COMPANY OVERVIEW
23.7.2 REVENUE ANALYSIS
23.7.3 GEOGRAPHIC PRESENCE
23.7.4 PRODUCT PORTFOLIO
23.7.5 RECENT DEVELOPMENTS
23.8 DSM-FIRMENICH
23.8.1 COMPANY OVERVIEW
23.8.2 REVENUE ANALYSIS
23.8.3 GEOGRAPHIC PRESENCE
23.8.4 PRODUCT PORTFOLIO
23.8.5 RECENT DEVELOPMENTS
23.9 DUPONT
23.9.1 COMPANY OVERVIEW
23.9.2 REVENUE ANALYSIS
23.9.3 GEOGRAPHIC PRESENCE
23.9.4 PRODUCT PORTFOLIO
23.9.5 RECENT DEVELOPMENTS
23.1 YAKULT HONSHA CO., LTD.
23.10.1 COMPANY OVERVIEW
23.10.2 REVENUE ANALYSIS
23.10.3 GEOGRAPHIC PRESENCE
23.10.4 PRODUCT PORTFOLIO
23.10.5 RECENT DEVELOPMENTS
23.11 INGREDION
23.11.1 COMPANY OVERVIEW
23.11.2 REVENUE ANALYSIS
23.11.3 GEOGRAPHIC PRESENCE
23.11.4 PRODUCT PORTFOLIO
23.11.5 RECENT DEVELOPMENTS
23.12 SYNLOGICS
23.12.1 COMPANY OVERVIEW
23.12.2 REVENUE ANALYSIS
23.12.3 GEOGRAPHIC PRESENCE
23.12.4 PRODUCT PORTFOLIO
23.12.5 RECENT DEVELOPMENTS
23.13 SERES THERAPEUTICS
23.13.1 COMPANY OVERVIEW
23.13.2 REVENUE ANALYSIS
23.13.3 GEOGRAPHIC PRESENCE
23.13.4 PRODUCT PORTFOLIO
23.13.5 RECENT DEVELOPMENTS
23.14 MICROBIOTICA
23.14.1 COMPANY OVERVIEW
23.14.2 REVENUE ANALYSIS
23.14.3 GEOGRAPHIC PRESENCE
23.14.4 PRODUCT PORTFOLIO
23.14.5 RECENT DEVELOPMENTS
23.15 REBIOTIX INC. (FERRING)
23.15.1 COMPANY OVERVIEW
23.15.2 REVENUE ANALYSIS
23.15.3 GEOGRAPHIC PRESENCE
23.15.4 PRODUCT PORTFOLIO
23.15.5 RECENT DEVELOPMENTS
23.16 VEDANTA BIOSCIENCES, INC
23.16.1 COMPANY OVERVIEW
23.16.2 REVENUE ANALYSIS
23.16.3 GEOGRAPHIC PRESENCE
23.16.4 PRODUCT PORTFOLIO
23.16.5 RECENT DEVELOPMENTS
23.17 SIOLTA THERAPEUTICS
23.17.1 COMPANY OVERVIEW
23.17.2 REVENUE ANALYSIS
23.17.3 GEOGRAPHIC PRESENCE
23.17.4 PRODUCT PORTFOLIO
23.17.5 RECENT DEVELOPMENTS
23.18 KANVAS BIOSCIENCES
23.18.1 COMPANY OVERVIEW
23.18.2 REVENUE ANALYSIS
23.18.3 GEOGRAPHIC PRESENCE
23.18.4 PRODUCT PORTFOLIO
23.18.5 RECENT DEVELOPMENTS
23.19 MICROBIOME THERAPEUTICS INNOVATION GROUP
23.19.1 COMPANY OVERVIEW
23.19.2 REVENUE ANALYSIS
23.19.3 GEOGRAPHIC PRESENCE
23.19.4 PRODUCT PORTFOLIO
23.19.5 RECENT DEVELOPMENTS
23.2 AZITRA
23.20.1 COMPANY OVERVIEW
23.20.2 REVENUE ANALYSIS
23.20.3 GEOGRAPHIC PRESENCE
23.20.4 PRODUCT PORTFOLIO
23.20.5 RECENT DEVELOPMENTS
23.21 CHR. HANSEN HOLDING A/S
23.21.1 COMPANY OVERVIEW
23.21.2 REVENUE ANALYSIS
23.21.3 GEOGRAPHIC PRESENCE
23.21.4 PRODUCT PORTFOLIO
23.21.5 RECENT DEVELOPMENTS
23.22 RITTER PHARMACEUTICALS
23.22.1 COMPANY OVERVIEW
23.22.2 REVENUE ANALYSIS
23.22.3 GEOGRAPHIC PRESENCE
23.22.4 PRODUCT PORTFOLIO
23.22.5 RECENT DEVELOPMENTS
23.23 PHARMAVITE
23.23.1 COMPANY OVERVIEW
23.23.2 REVENUE ANALYSIS
23.23.3 GEOGRAPHIC PRESENCE
23.23.4 PRODUCT PORTFOLIO
23.23.5 RECENT DEVELOPMENTS
23.24 SYMBIOTIX BIOTHERAPIES, INC.
23.24.1 COMPANY OVERVIEW
23.24.2 REVENUE ANALYSIS
23.24.3 GEOGRAPHIC PRESENCE
23.24.4 PRODUCT PORTFOLIO
23.24.5 RECENT DEVELOPMENTS
23.25 MICROBIOTICA
23.25.1 COMPANY OVERVIEW
23.25.2 REVENUE ANALYSIS
23.25.3 GEOGRAPHIC PRESENCE
23.25.4 PRODUCT PORTFOLIO
23.25.5 RECENT DEVELOPMENTS
23.26 QUORUM INNOVATIONS
23.26.1 COMPANY OVERVIEW
23.26.2 REVENUE ANALYSIS
23.26.3 GEOGRAPHIC PRESENCE
23.26.4 PRODUCT PORTFOLIO
23.26.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
24 RELATED REPORTS
25 QUESTIONNAIRE
26 CONCLUSION
27 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.