Global Microvillus Inclusion Disease Market
Market Size in USD Billion
CAGR :
% 
 USD
1.60 Billion
USD
2.19 Billion
2024
2032
USD
1.60 Billion
USD
2.19 Billion
2024
2032
| 2025 –2032 | |
| USD 1.60 Billion | |
| USD 2.19 Billion | |
|
|
|
|
Microvillus Inclusion Disease Market Size
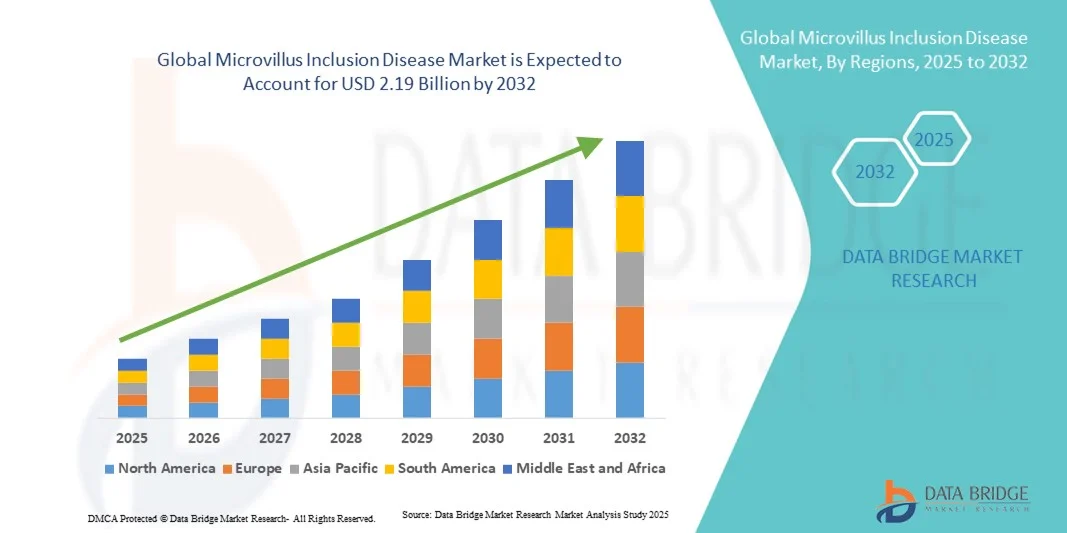
- The global microvillus inclusion disease market size was valued at USD 1.60 billion in 2024 and is expected to reach USD 2.19 billion by 2032, at a CAGR of 4.00% during the forecast period
- The market growth is largely driven by rising research efforts into rare gastrointestinal disorders and advancements in genetic diagnostics, which are improving early detection and treatment strategies for microvillus inclusion disease (MVID)
- Furthermore, increasing awareness among healthcare professionals, growing support from rare disease foundations, and ongoing development of innovative gene and stem cell therapies are fostering progress in this field. These converging factors are accelerating clinical research initiatives, thereby significantly boosting the industry's growth
Microvillus Inclusion Disease Market Analysis
- Microvillus Inclusion Disease (MVID), a rare congenital enteropathy characterized by severe neonatal diarrhea and nutrient malabsorption, is increasingly recognized within the global rare disease therapeutics landscape due to advancements in molecular diagnostics and precision medicine
- The growing demand for accurate diagnosis and improved patient management is primarily fueled by progress in next-generation sequencing (NGS), increased clinical awareness, and the establishment of specialized pediatric gastroenterology and genetic counseling centers
- North America dominated the microvillus inclusion disease market with the largest revenue share of 42.8% in 2024, supported by strong healthcare infrastructure, advanced diagnostic technologies, and active research collaborations focused on understanding genetic mutations such as MYO5B linked to MVID
- Asia-Pacific is expected to be the fastest-growing region in the microvillus inclusion disease market during the forecast period, driven by improving healthcare access, growing investment in rare disease research, and expanding adoption of genomic testing in countries such as Japan, China, and India
- The genetic testing segment dominated the market with a share of 56.9% in 2024, owing to its essential role in confirming diagnosis through mutation detection, enabling targeted disease management and facilitating early intervention strategies for affected infants
Report Scope and Microvillus Inclusion Disease Market Segmentation
|
Attributes |
Microvillus Inclusion Disease Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Microvillus Inclusion Disease Market Trends
Advancements in Gene Therapy and Precision Diagnostics
- A significant and accelerating trend in the global microvillus inclusion disease (MVID) market is the growing focus on gene therapy and advanced molecular diagnostics, aimed at addressing the root genetic mutations responsible for the disease rather than only managing symptoms
- For instance, ongoing research on MYO5B gene correction using CRISPR and AAV-based delivery systems is gaining traction, as scientists explore personalized therapeutic strategies for this rare gastrointestinal disorder. Similarly, institutions such as the National Institutes of Health (NIH) are conducting genetic studies to enhance understanding of disease mechanisms
- Gene-targeted therapy development enables precision medicine approaches that hold promise for long-term disease management by restoring normal enterocyte function, potentially reducing dependency on parenteral nutrition. For instance, advancements in organoid and stem cell models allow researchers to evaluate gene-editing outcomes before clinical translation
- The integration of molecular diagnostics, biobanking, and next-generation sequencing (NGS) platforms facilitates centralized data collection for patient characterization, early detection, and therapy response assessment. Through such frameworks, clinicians can manage patient care more effectively and accelerate rare disease trials
- This trend toward targeted, personalized, and research-driven treatment development is fundamentally reshaping therapeutic innovation in the MVID market. Consequently, companies and research organizations are expanding R&D efforts to establish viable gene and cell-based solutions that may provide durable clinical benefits
- The demand for gene therapy and precision diagnostics in MVID is growing rapidly across global research institutions and biotech firms, as stakeholders increasingly prioritize translational genomics and advanced molecular interventions for rare pediatric diseases
Microvillus Inclusion Disease Market Dynamics
Driver
Growing Research Investments and Advancements in Genetic Diagnostics
- The increasing investment in rare disease research programs, coupled with technological advancements in genetic sequencing and molecular testing, is a key driver fueling growth in the microvillus inclusion disease market
- For instance, in March 2024, the European Rare Disease Research Alliance initiated a collaborative project focused on developing novel genetic screening methods and potential gene-based treatments for congenital enteropathies such as MVID. Such programs are expected to drive clinical innovation in the forecast period
- As healthcare systems enhance genomic testing infrastructure, early detection of MYO5B mutations is becoming more feasible, improving diagnosis rates and enabling precision-based patient management strategies
- Furthermore, the increasing collaboration between academic institutions, biotechnology companies, and non-profit organizations is promoting the development of specialized diagnostic assays and therapeutic candidates for ultra-rare disorders
- The focus on improving diagnostic yield through next-generation sequencing, combined with funding incentives for orphan disease research, is propelling growth across key regions. Technological progress in molecular imaging and data-driven clinical workflows further strengthens the diagnostic ecosystem
- The trend toward patient registries, data sharing, and multidisciplinary research frameworks continues to expand, supporting knowledge exchange and clinical advancement across the global MVID research landscape
Restraint/Challenge
Limited Patient Population and High Research Costs
- The rarity of microvillus inclusion disease significantly limits the availability of large patient cohorts, posing a major challenge for clinical trials, data validation, and commercial viability of targeted therapies
- For instance, the small number of confirmed global cases, mostly identified through neonatal screening in specialized centers, makes patient recruitment for advanced therapeutic studies highly challenging and time-consuming
- Addressing this limitation through the creation of international patient registries and centralized biobanks is critical for advancing translational research and developing effective treatment pathways. Companies and research groups emphasize collaborations to pool clinical data and genetic samples. In addition, the high cost of gene and cell-based therapy development for ultra-rare disorders remains a barrier to scalability and accessibility
- While ongoing research offers promise, the financial burden of sustaining long-term R&D initiatives with minimal patient pools discourages some biopharma entities from investing in this niche market
- The complexity of regulatory approval processes for orphan therapies, coupled with the need for robust safety validation, further prolongs development timelines and increases operational costs. This presents a substantial hurdle for emerging biotech firms entering the field
- Overcoming these challenges through international collaboration, targeted funding programs, and policy incentives for rare disease innovation will be vital to unlock the full therapeutic potential of the microvillus inclusion disease market
Microvillus Inclusion Disease Market Scope
The market is segmented on the basis of related disorders and diagnosis.
- By Related Disorders
On the basis of related disorders, the global microvillus inclusion disease (MVID) market is segmented into Lactose Intolerance, Familial Chloride Diarrhoea or Congenital Chloride Diarrhoea (CCD), and Infantile Diarrhoea with Abnormal Hair. Familial Chloride Diarrhoea or Congenital Chloride Diarrhoea (CCD) segment dominated the market with the largest market revenue share of 46.8% in 2024, driven by its close clinical and genetic association with MVID and the growing number of diagnostic screenings identifying shared pathogenic mechanisms. CCD, a rare autosomal recessive disorder characterized by chronic watery diarrhea, shares similar intestinal transport dysfunctions with MVID, leading to overlapping research focus and treatment development. Increasing advancements in molecular genetics and neonatal screening have significantly improved CCD detection, allowing earlier differentiation from MVID and other congenital diarrheal disorders. Furthermore, the active inclusion of CCD cases in rare gastrointestinal disease registries has strengthened clinical research collaborations and accelerated therapy evaluation across both conditions.
The Infantile Diarrhoea with Abnormal Hair segment is anticipated to witness the fastest growth rate of 8.9% from 2025 to 2032, fueled by expanding research into syndromic enteropathies with shared molecular pathways. This disorder, often linked with trichohepatoenteric syndrome, is receiving growing scientific attention for its genetic overlaps with MVID, particularly concerning epithelial cell polarity and brush border formation. Increasing genomic testing and phenotype-based diagnosis are leading to earlier recognition and accurate differentiation of these rare syndromes in infants. Moreover, collaborative research efforts between pediatric gastroenterologists and molecular biologists are advancing understanding of related enteropathies, driving innovation in targeted treatment and genetic counseling services.
- By Diagnosis
On the basis of diagnosis, the microvillus inclusion disease market is segmented into genetic testing and others. The Genetic Testing segment dominated the market with the largest revenue share of 56.9% in 2024, owing to its crucial role in confirming MVID diagnosis by identifying pathogenic mutations in the MYO5B, STX3, or STXBP2 genes. Advancements in next-generation sequencing (NGS), whole-exome sequencing (WES), and targeted genetic panels have significantly improved diagnostic accuracy and reduced the time to definitive identification. Growing clinical awareness and increasing integration of molecular diagnostics in neonatal intensive care units (NICUs) are also boosting adoption. Genetic testing not only enables early detection but also supports family genetic counseling, allowing for better clinical decision-making and future prenatal screening initiatives.
The Others segment, which includes histopathological analysis, electron microscopy, and clinical evaluation, is expected to witness the fastest growth rate of 7.8% from 2025 to 2032, driven by its continued importance in confirming disease pathology when genetic results are inconclusive. Electron microscopy remains a gold standard for visualizing microvillus inclusions within enterocytes, providing direct morphological evidence of disease presence. Increasing access to specialized diagnostic laboratories and advancements in tissue imaging technology are improving diagnostic yield for rare enteropathies. Furthermore, combined approaches integrating histology with immunostaining and molecular profiling are enhancing diagnostic precision, supporting more comprehensive evaluation of MVID and related disorders across research and clinical settings.
Microvillus Inclusion Disease Market Regional Analysis
- North America dominated the microvillus inclusion disease market with the largest revenue share of 42.8% in 2024, supported by strong healthcare infrastructure, advanced diagnostic technologies, and active research collaborations focused on understanding genetic mutations such as MYO5B linked to MVID
- Clinicians and researchers in the region increasingly emphasize early genetic diagnosis, precision medicine, and improved patient management through collaboration among pediatric gastroenterology and molecular genetics centers
- This regional leadership is further supported by robust funding for orphan disease research, active participation of academic institutions and biotech firms in clinical trials, and strong governmental and non-profit support for neonatal and genetic screening programs, positioning North America as the global hub for MVID diagnostics and research advancement
U.S. Microvillus Inclusion Disease Market Insight
The U.S. microvillus inclusion disease (MVID) market captured the largest revenue share of 79% in 2024 within North America, driven by strong clinical research networks, advanced genetic testing infrastructure, and early adoption of precision diagnostics. Increasing awareness of rare gastrointestinal disorders among healthcare professionals and improved access to next-generation sequencing (NGS) technologies are accelerating early disease detection. The growing number of NIH-funded research initiatives and collaborations between pediatric hospitals and biotech firms further strengthen the market. Moreover, favorable regulatory pathways for orphan drugs and patient support programs are propelling continued investment and innovation in the U.S. MVID landscape.
Europe Microvillus Inclusion Disease Market Insight
The Europe microvillus inclusion disease market is projected to expand at a steady CAGR throughout the forecast period, primarily supported by active participation in cross-border rare disease research initiatives and growing investment in genetic diagnostics. European nations are focusing on early identification and genetic counseling for congenital enteropathies through well-established healthcare systems. The region’s coordinated rare disease strategies, supported by the European Medicines Agency (EMA), enhance therapeutic development and patient data harmonization. The inclusion of MVID in national rare disease registries and increased funding for advanced diagnostic technologies continue to foster clinical progress across the continent.
U.K. Microvillus Inclusion Disease Market Insight
The U.K. microvillus inclusion disease market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by strong genetic research capabilities and national initiatives promoting early rare disease diagnosis. The NHS Genomic Medicine Service plays a crucial role in enabling comprehensive screening for congenital gastrointestinal conditions such as MVID. Increasing collaboration between universities, clinical centers, and biotech firms is further driving diagnostic innovation and therapy exploration. In addition, government-backed funding and awareness programs are improving access to care and supporting the development of precision medicine approaches for ultra-rare disorders.
Germany Microvillus Inclusion Disease Market Insight
The Germany microvillus inclusion disease market is expected to expand at a considerable CAGR during the forecast period, fueled by advancements in molecular diagnostics and high levels of healthcare R&D investment. The country’s robust biotechnology ecosystem and focus on translational medicine are propelling research into novel treatment modalities for genetic enteropathies such as MVID. German academic institutions are actively participating in European rare disease consortiums, facilitating patient registry integration and genetic data exchange. The growing adoption of next-generation sequencing and biobanking technologies supports early diagnosis and fosters long-term patient monitoring programs.
Asia-Pacific Microvillus Inclusion Disease Market Insight
The Asia-Pacific microvillus inclusion disease market is poised to grow at the fastest CAGR of 8.7% during the forecast period of 2025 to 2032, driven by improving healthcare infrastructure, rising awareness of rare diseases, and expanding access to genetic testing. Countries such as Japan, China, and India are witnessing rapid adoption of molecular diagnostics in neonatal care. Government-led initiatives supporting genomic research and the establishment of rare disease networks are strengthening regional diagnostic capacity. Furthermore, the growing collaboration between Asian research institutions and global biotech firms is enhancing clinical trial participation and access to advanced therapeutic research in MVID.
Japan Microvillus Inclusion Disease Market Insight
The Japan microvillus inclusion disease market is gaining momentum due to the country’s highly advanced biotechnology sector and commitment to precision medicine. Increased investments in pediatric genetic research and the integration of MVID screening into neonatal programs are accelerating diagnosis. Japan’s robust healthcare policies and strong academic-industry collaborations promote the development of new therapeutic approaches for rare congenital disorders. Moreover, the emphasis on technological innovation, such as the use of stem cell and organoid models, is contributing to advancements in disease modeling and personalized treatment development.
India Microvillus Inclusion Disease Market Insight
The India microvillus inclusion disease market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to growing awareness of rare diseases, expanding access to molecular diagnostics, and increasing government focus on healthcare modernization. India’s emerging biotechnology sector and the establishment of genomic testing centers are improving early detection rates. The country’s participation in global rare disease research programs and its growing pool of skilled geneticists are supporting advancements in MVID diagnosis and care. In addition, national health initiatives promoting rare disease management and affordable testing solutions are expected to drive continued market expansion.
Microvillus Inclusion Disease Market Share
The Microvillus Inclusion Disease industry is primarily led by well-established companies, including:
- Jaguar Health, Inc. (U.S.)
- Napo Therapeutics S.p.A (Italy)
- Illumina, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- BGI Group (China)
- Labcorp (U.S.)
- Eurofins Scientific SE (Luxembourg)
- QIAGEN (Netherlands)
- Agilent Technologies, Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- PerkinElmer (U.S.)
- Charles River Laboratories (U.S.)
- Revvity, Inc. (U.S.)
- Centogene N.V. (Germany)
- GeneDx, LLC (U.S.)
- 10x Genomics, Inc. (U.S.)
- Twist Bioscience (U.S.)
- Oxford Nanopore Technologies plc. (U.K.)
- PacBio. (U.S.)
What are the Recent Developments in Global Microvillus Inclusion Disease Market?
- In September 2025, Jaguar Health announced that the FDA had authorized expanded access programs (EAPs) utilizing Crofelemer powder for oral solution to treat two pediatric intestinal failure patients suffering from MVID. These single-patient INDs allowed compassionate use of the investigational therapy for critically ill children outside clinical trials
- In December 2024, Jaguar Health officially initiated a Phase 2 clinical study evaluating Crofelemer in pediatric patients diagnosed with MVID. This clinical trial aimed to assess safety, dosing, and efficacy of the drug in managing congenital secretory diarrheas. The study represented one of the first structured, multi-site clinical evaluations for MVID globally, offering hope for a potential disease-modifying treatment in a condition where therapeutic options remain extremely limited
- In May 2024, Jaguar Health’s family of companies including Napo Pharmaceuticals and Napo Therapeutics announced the submission of Clinical Trial Applications (CTAs) to health authorities in Italy, Germany, and other regions across Europe and MENA. These submissions aimed to initiate human clinical trials for Crofelemer oral powder in MVID and short bowel syndrome with intestinal failure (SBS-IF)
- In June 2023, Napo Pharmaceuticals, Inc., a subsidiary of Jaguar Health, announced that it had submitted an Investigational New Drug (IND) application to the FDA for a new powder formulation of Crofelemer designed specifically for the treatment of MVID. This development represented a major step toward initiating clinical trials in infants and children who suffer from chronic, life-threatening diarrhea caused by the disease. The novel formulation was tailored for pediatric administration, improving usability and absorption in neonatal patients
- In February 2023, Jaguar Health, Inc. announced that its proprietary antidiarrheal drug Crofelemer received Orphan Drug Designation (ODD) from the U.S. Food and Drug Administration (FDA) for the treatment of Microvillus Inclusion Disease (MVID). This milestone recognized Crofelemer’s potential to address the high unmet medical need associated with this ultra-rare pediatric disorder. The ODD status offers benefits such as market exclusivity and regulatory support, positioning Jaguar Health as a leading developer in the MVID therapeutic space
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.