Global Mitochondrial Neurogastrointestinal Encephalomyopathy Mngie Market
Market Size in USD Billion
CAGR :
% 
 USD
2.50 Billion
USD
3.16 Billion
2024
2032
USD
2.50 Billion
USD
3.16 Billion
2024
2032
| 2025 –2032 | |
| USD 2.50 Billion | |
| USD 3.16 Billion | |
|
|
|
|
Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Size
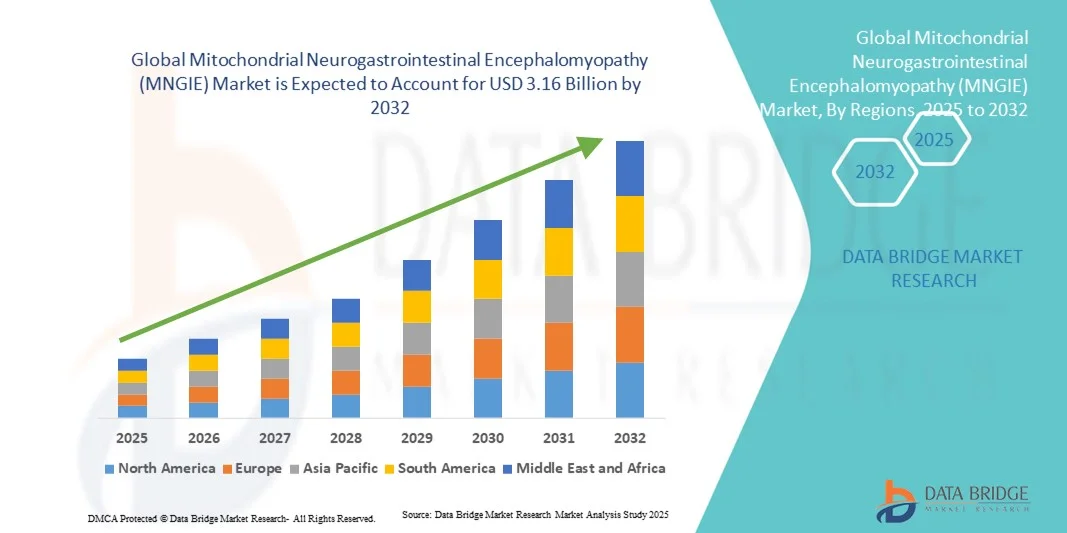
- The global Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) market size was valued at USD 2.50 billion in 2024 and is expected to reach USD 3.16 billion by 2032, at a CAGR of 3.00% during the forecast period
- The market growth is largely fueled by the increasing advancements in genetic research, mitochondrial medicine, and targeted therapeutic development, which are significantly enhancing the understanding and treatment of rare mitochondrial disorders such as Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE). The growing focus on precision medicine and gene therapy is leading to greater innovation in diagnostic and treatment approaches, improving patient outcomes and supporting the expansion of this niche therapeutic area
- Furthermore, rising global awareness about rare diseases, growing investments from biotechnology companies in orphan drug development, and increasing collaborations between research institutions and pharmaceutical firms are accelerating the progress of clinical studies for MNGIE. These converging factors are driving the adoption of advanced mitochondrial-targeted therapies and enzyme replacement strategies, thereby significantly boosting the overall growth of the Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) market
Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Analysis
- Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE), a rare autosomal recessive mitochondrial disorder, is gaining increased clinical and research attention due to growing advancements in mitochondrial biology, enzyme replacement therapy, and gene therapy. The disease’s complex nature involving both neurological and gastrointestinal manifestations has encouraged deeper research into targeted treatment approaches and diagnostic innovations
- The rising prevalence of mitochondrial disorders, coupled with expanding genetic testing capabilities and early diagnosis initiatives, is driving the global demand for specialized therapies addressing MNGIE. Furthermore, ongoing clinical trials, increasing research collaborations, and support from rare disease foundations are fueling market expansion
- North America dominated the mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market with the largest revenue share of 42.3% in 2024, driven by advanced healthcare infrastructure, strong government support for orphan drug development, and the presence of key biopharmaceutical companies investing in mitochondrial disease research. The U.S. continues to lead due to growing patient awareness, access to genomic testing, and a high number of ongoing clinical trials targeting mitochondrial dysfunction
- Asia-Pacific is expected to be the fastest-growing region, registering a CAGR of 9.7% during the forecast period, attributed to improving healthcare expenditure, expanding access to advanced diagnostic tools, and the increasing participation of regional research institutions in mitochondrial disease studies. Rising collaborations between biotech firms and academic centers are also contributing to faster therapeutic innovation across countries like Japan, China, and South Korea
- The drug therapies segment accounted for the largest market revenue share of 63.1% in 2024, primarily due to the widespread use of enzyme replacement and nucleoside reduction therapies
Report Scope and Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Segmentation
|
Attributes |
Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Trends
Enhanced Diagnosis and Therapeutic Advancements through AI and Genomic Integration
- A significant and accelerating trend in the global mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market is the integration of artificial intelligence (AI), advanced genomic sequencing, and digital health analytics for improved disease diagnosis, monitoring, and therapeutic discovery. This technological convergence is revolutionizing the understanding and management of rare mitochondrial disorders by enhancing diagnostic precision and facilitating the identification of novel biomarkers
- For instance, AI-assisted genomic interpretation platforms are being increasingly utilized in clinical laboratories to identify TYMP and POLG gene mutations responsible for MNGIE with greater accuracy and speed. Similarly, digital phenotyping tools supported by AI algorithms are enabling clinicians to analyze patient-reported data and metabolic patterns to detect early disease progression
- Furthermore, the adoption of AI-driven drug discovery pipelines allows pharmaceutical companies to simulate mitochondrial enzyme deficiencies and predict the efficacy of candidate molecules before clinical testing. These tools also aid in optimizing dosing strategies for emerging nucleoside metabolism modulators. The integration of AI and genomics is not only enhancing early diagnosis but also enabling precision medicine by aligning treatment protocols to specific genetic variants
- The increasing use of telemedicine and digital patient registries is further streamlining disease monitoring, facilitating real-time data exchange among clinicians, and fostering global collaboration in research. These advancements are fundamentally transforming clinical workflows, improving diagnostic yield, and reshaping patient expectations for timely and effective care. Consequently, biotech firms and academic institutions are focusing on AI-powered research collaborations to accelerate gene-based therapy development and patient outcome optimization
- The rising demand for AI-assisted diagnostics and digital genomic interpretation is growing rapidly across both hospital and research settings, as stakeholders increasingly prioritize precision, speed, and personalization in rare disease management
Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Dynamics
Driver
Growing Need for Targeted Therapies and Increasing Awareness of Rare Mitochondrial Disorders
- The rising global prevalence of rare mitochondrial disorders and growing awareness about genetic conditions among clinicians and patients are major drivers for the MNGIE market’s expansion
- For instance, in April 2024, several research institutions announced progress in enzyme replacement therapy and gene-editing studies for MNGIE, highlighting the industry’s growing investment in curative treatment approaches. Such initiatives by biotechnology and academic collaborations are expected to drive market growth throughout the forecast period
- Increasing diagnostic testing availability, combined with supportive government policies for rare disease research and funding, has accelerated early detection and intervention efforts. Advanced molecular diagnostics, including next-generation sequencing and metabolite profiling, are being increasingly adopted in clinical practice to confirm MNGIE diagnosis
- Furthermore, patient advocacy organizations and global rare disease networks are contributing to early awareness campaigns, promoting genetic counseling, and improving patient access to experimental therapies through compassionate-use programs
- The rising focus on precision medicine, personalized therapy design, and integration of multidisciplinary care models—including neurology, gastroenterology, and metabolic specialists—is further propelling the MNGIE market’s growth
Restraint/Challenge
High Treatment Costs and Limited Clinical Infrastructure for Rare Disease Management
- High treatment costs and limited specialized infrastructure for rare disease management pose significant challenges to the broader adoption of emerging MNGIE therapies. The complex nature of mitochondrial disorders requires advanced diagnostic facilities, specialized clinicians, and genetic counseling services—resources often unavailable in low- and middle-income regions
- For instance, limited access to enzyme replacement therapies, gene therapy trials, and nucleoside metabolism modulators in developing countries has restricted patient inclusion in clinical research and delayed treatment initiation
- Moreover, the high cost of gene-based therapies and long-term patient monitoring makes these treatments inaccessible to a large portion of the global population, despite ongoing reimbursement policy improvements in some regions
- Concerns related to the stability, safety, and delivery efficiency of mitochondrial-targeted therapies also present technical barriers to commercialization
- Addressing these challenges requires increased public–private collaboration, establishment of global diagnostic centers of excellence, and development of affordable gene-editing and enzyme replacement options. Enhanced healthcare infrastructure, coupled with education and financial support mechanisms, will be vital for ensuring equitable access and sustained market expansion
Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Scope
The market is segmented on the basis of symptoms, treatment, route of administration, and distribution channel.
- By Symptoms
On the basis of symptoms, the Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) market is segmented into external ophthalmoplegia, gastrointestinal dysmotility, cachexia, peripheral neuropathy, and leukoencephalopathy. The gastrointestinal dysmotility segment dominated the largest market revenue share of 38.4% in 2024, owing to the high frequency of severe digestive complications in patients suffering from MNGIE. This symptom affects both nutrient absorption and gastrointestinal movement, often leading to malnutrition and chronic abdominal discomfort. Hospitals and clinics are increasingly prioritizing management of these symptoms through enzyme replacement and dietary interventions. In addition, early recognition of gastrointestinal issues via genetic and metabolic testing is improving diagnosis rates. Growing awareness about rare mitochondrial disorders and expansion of research programs focused on gut-mitochondrial connections are further boosting clinical management. The availability of supportive care therapies and multidisciplinary treatment centers has also reinforced this segment’s dominance within the global market.
The external ophthalmoplegia segment is projected to register the fastest growth rate, with a CAGR of 21.2% from 2025 to 2032, fueled by the increasing development of ocular-targeted gene therapies and improved mitochondrial imaging technologies. Early detection through advanced ophthalmic screening and molecular diagnostics is enhancing treatment accuracy. Research on mitochondrial transfer and gene editing is showing promise in correcting ocular muscle weakness. The growing prevalence of visual disturbances in mitochondrial diseases has raised awareness among neurologists and ophthalmologists alike. Moreover, collaborations between biotech firms and academic institutes are driving innovation in ocular mitochondrial treatments. Rising clinical trials focusing on vision restoration are expected to further accelerate this segment’s growth over the forecast period.
- By Treatment
On the basis of treatment, the MNGIE market is segmented into drug therapies and occupational and physical therapy. The drug therapies segment accounted for the largest market revenue share of 63.1% in 2024, primarily due to the widespread use of enzyme replacement and nucleoside reduction therapies. These therapies directly address the underlying thymidine phosphorylase enzyme deficiency responsible for the disease. Pharmaceutical companies are investing heavily in mitochondrial-targeted therapeutics and supportive medications. Increased government and private funding for orphan drug development has also expanded patient access. Advances in drug formulation, along with regulatory approvals for compassionate use programs, have significantly supported growth. In addition, expanding hospital pharmacy channels and clinical programs for mitochondrial disorders have strengthened this segment’s position in the global market.
The occupational and physical therapy segment is expected to witness the fastest CAGR of 20.8% from 2025 to 2032, driven by the rising demand for rehabilitative care to improve motor skills and muscle coordination in MNGIE patients. Rehabilitation programs play a crucial role in maintaining patient independence and enhancing overall quality of life. Growing investments in specialized therapy centers and increased inclusion of physiotherapy in rare disease care models are supporting market expansion. The adoption of technology-driven rehabilitation tools, such as wearable movement sensors and tele-therapy platforms, further strengthens accessibility. Collaboration between rehabilitation experts and neurologists is also improving therapy outcomes. This increasing integration of physical and occupational therapy into long-term patient care is projected to fuel rapid segmental growth through the forecast period.
- By Route of Administration
On the basis of route of administration, the MNGIE market is segmented into oral and injection. The oral segment dominated the market with the largest revenue share of 57.6% in 2024, attributed to strong patient preference for non-invasive treatment options and ease of administration. Oral formulations are particularly beneficial for long-term therapy, allowing patients to manage symptoms conveniently at home. The development of oral mitochondrial stabilizers and enzyme-modulating compounds has contributed to this dominance. Moreover, increasing awareness of mitochondrial function and availability of oral nucleoside-modifying drugs are propelling growth. Enhanced focus on patient adherence and comfort has further reinforced oral route adoption. Hospitals and pharmacies globally are witnessing rising demand for oral formulations as part of integrated MNGIE care management.
The injection segment is projected to record the fastest CAGR of 22.4% from 2025 to 2032, due to the rising adoption of intravenous enzyme replacement and mitochondrial gene therapies. Injectable formulations offer precise dosing and faster therapeutic outcomes, which are essential in patients with severe gastrointestinal dysfunction where oral absorption is limited. The growing number of hospital-based infusion centers and patient support programs for rare disease treatments are contributing to this growth. Biopharmaceutical advancements in enzyme stabilization and recombinant formulations have improved treatment efficacy. The expansion of targeted drug delivery systems and increased acceptance of hospital-administered therapies are further expected to drive this segment’s rapid growth across the forecast period.
- By Distribution Channel
On the basis of distribution channel, the MNGIE market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the largest revenue share of 49.2% in 2024, supported by centralized distribution and controlled administration of specialized mitochondrial therapies. Hospitals act as key hubs for MNGIE management, providing comprehensive diagnostic and treatment solutions. The presence of dedicated rare disease departments and infusion centers ensures availability of enzyme replacement and supportive therapies. Collaboration between hospitals and pharmaceutical firms for orphan drug access has enhanced therapeutic reach. Moreover, increased patient admissions for intravenous therapies and clinical monitoring has bolstered hospital pharmacy sales. Strong clinical governance, drug safety standards, and direct physician involvement further reinforce this segment’s leading position in the market.
The online pharmacy segment is anticipated to witness the fastest growth rate, registering a CAGR of 23.1% from 2025 to 2032, owing to rising digital healthcare penetration and patient preference for convenient, home-based drug delivery. The expansion of e-pharmacy networks offering specialized rare disease medications is improving global accessibility. Online platforms provide price transparency, prescription validation, and data tracking features, ensuring reliability and ease for patients. Strategic partnerships between biotech manufacturers and online distributors are increasing therapy availability across regions. Moreover, the rise of telehealth consultations and government approvals for e-prescriptions have strengthened digital distribution channels. Growing trust in online platforms among rare disease patients is expected to sustain this segment’s rapid growth through the forecast period.
Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Regional Analysis
- North America dominated the mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market with the largest revenue share of 42.3% in 2024, driven by advanced healthcare infrastructure, strong government support for orphan drug development, and the presence of key biopharmaceutical companies investing in mitochondrial disease research
- The region benefits from extensive clinical research networks, access to genomic testing, and favorable reimbursement frameworks supporting the diagnosis and treatment of rare disorders such as MNGIE
- Growing collaborations between hospitals, academic centers, and biotech firms are accelerating the development of targeted enzyme replacement therapies and gene-based interventions. Furthermore, increased awareness among healthcare professionals and patients, coupled with expanding diagnostic screening programs, has significantly contributed to early identification and management of mitochondrial disorders across the region
U.S. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Insight
The U.S. mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market captured the largest revenue share in 2024 within North America, primarily due to the high prevalence of genetic testing and the strong presence of specialized treatment centers focused on mitochondrial medicine. The country’s advanced clinical infrastructure and active research in enzyme and gene replacement therapies continue to drive innovation. Moreover, favorable regulatory incentives such as the Orphan Drug Act, coupled with the support of patient advocacy groups, have encouraged biotech firms to invest in novel therapeutic solutions. The U.S. also benefits from a robust pipeline of investigational drugs and clinical trials aimed at improving outcomes for patients with mitochondrial dysfunction.
Europe Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Insight
The Europe mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market is projected to expand at a significant CAGR throughout the forecast period, driven by strong research funding, growing awareness of rare mitochondrial diseases, and the presence of established genetic testing infrastructure. The European Medicines Agency’s regulatory support for orphan drug designation is encouraging regional biotechnology companies to pursue clinical development for enzyme and gene replacement therapies. Increased collaboration between national research institutes and hospitals is fostering innovation in diagnostic and treatment modalities. Moreover, patient advocacy organizations in countries such as Germany, France, and the U.K. are playing a vital role in promoting early diagnosis and access to emerging therapies.
U.K. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Insight
The U.K. mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by government initiatives such as the Genomics England Program, which focuses on enhancing rare disease diagnostics through advanced sequencing technologies. The National Health Service (NHS) provides structured support for genetic testing and patient management, improving accessibility to mitochondrial disorder care. In addition, academic collaborations between research universities and biotech companies are fostering translational research, while public awareness campaigns continue to encourage early testing and patient participation in clinical studies.
Germany Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Insight
The Germany mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market is expected to expand at a considerable CAGR during the forecast period, driven by the country’s strong biotechnology ecosystem, investment in rare disease research, and adoption of precision medicine approaches. Germany’s well-developed healthcare infrastructure supports comprehensive patient management, from genetic counseling to clinical intervention. The presence of leading pharmaceutical and academic research institutions conducting studies on mitochondrial dysfunction is accelerating therapeutic innovation. Furthermore, government-backed rare disease frameworks are improving diagnosis rates and promoting the development of affordable treatment options.
Asia-Pacific Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Insight
The Asia-Pacific mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market is poised to grow at the fastest CAGR of 9.7% during the forecast period of 2025 to 2032, attributed to increasing healthcare investments, expanding diagnostic capabilities, and the rising prevalence of inherited metabolic disorders. Rapid economic development in countries such as China, Japan, and South Korea is driving demand for advanced genetic testing and personalized medicine. In addition, regional participation in global clinical trials and partnerships with Western biotech firms are accelerating therapeutic advancements. Supportive government initiatives for rare disease management, along with the expansion of specialized research centers, are further enhancing market growth and accessibility across the region.
Japan Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Insight
The Japan mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market is gaining momentum due to the country’s strong focus on biomedical innovation, precision medicine, and early disease diagnosis. National policies promoting genomic medicine and integration of mitochondrial disease care within hospital systems are facilitating early detection. Japanese research organizations are actively engaged in clinical studies exploring enzyme replacement and gene therapy applications. In addition, collaborations between domestic biotech companies and universities are contributing to the discovery of new therapeutic pathways for MNGIE, supporting the country’s growing role in the global mitochondrial disease research landscape.
China Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Insight
The China mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) market accounted for the largest market revenue share in Asia-Pacific in 2024, supported by a growing middle-class population, increased healthcare spending, and strong investment in biotechnology innovation. The Chinese government’s emphasis on rare disease research and genetic medicine is driving advancements in mitochondrial disorder diagnostics and treatment. Local pharmaceutical companies are increasingly partnering with global firms to co-develop gene therapy and metabolic correction solutions. The establishment of regional genomics centers and biobanks is further promoting precision medicine initiatives, expanding accessibility to rare disease care across the country.
Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market Share
The Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) industry is primarily led by well-established companies, including:
- Pierrepont Therapeutics (U.S.)
- Neovii Biotech (Switzerland)
- Entrada Therapeutics (U.S.)
- Santhera Pharmaceuticals (Switzerland)
- Minovia Therapeutics (Israel)
- Stealth BioTherapeutics (U.S.)
- Reneo Pharmaceuticals (U.S.)
- Astellas Pharma (Japan)
- Reata Pharmaceuticals (U.S.)
- Khondrion (Netherlands)
- NeuroVive Pharmaceutical AB (Sweden)
- BridgeBio Pharma (U.S.)
- Mitobridge (U.S.)
- CohBar Inc. (U.S.)
Latest Developments in Global Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE) Market
- In July 2024, the European Medicines Agency (EMA) designated a novel therapeutic candidate for MNGIE as an orphan medicine under EU/3/24/2948, recognizing it for the treatment of thymidine-phosphorylase deficiency and enabling regulatory incentives for its development
- In September 2024, the Lily Foundation announced funding of a UK-based research team (University of Cambridge / AIBN) to develop an mRNA-based gene therapy for MNGIE, targeting TYMP enzyme replacement through advanced delivery systems in animal models
- In August 2025, a peer-reviewed article titled “New Horizons in the Treatment of MNGIE” highlighted upcoming therapeutic options — including liver transplantation, enzyme replacement therapy, and gene therapy — reflecting a shift toward curative rather than symptomatic approaches
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.