Global Monoclonal Antibody Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
263.22 Billion
USD
719.81 Billion
2024
2032
USD
263.22 Billion
USD
719.81 Billion
2024
2032
| 2025 –2032 | |
| USD 263.22 Billion | |
| USD 719.81 Billion | |
|
|
|
|
Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market Size
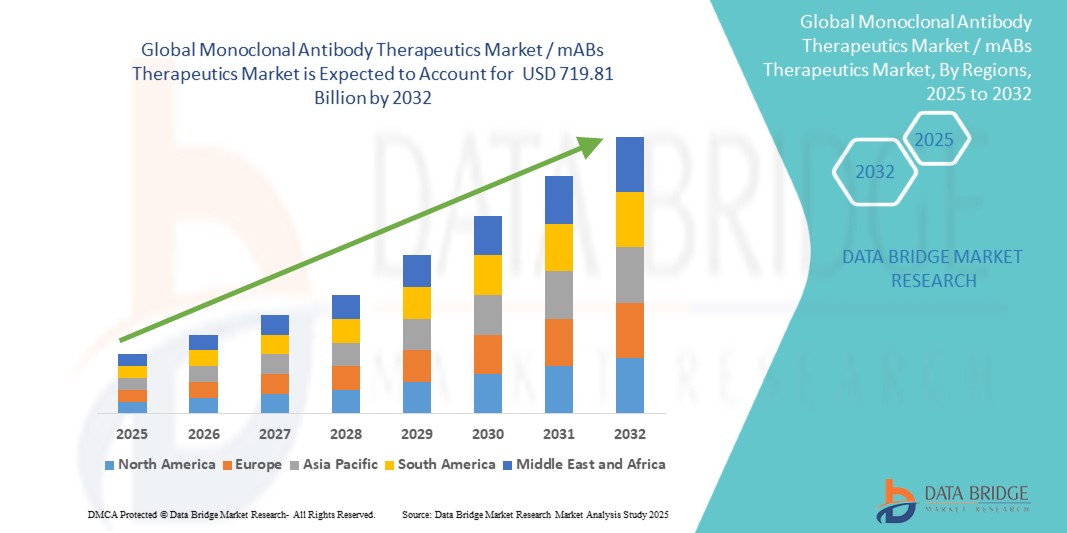
- The global monoclonal antibody therapeutics market / mABs therapeutics market size was valued at USD 263.22 billion in 2024 and is expected to reach USD 719.81 billion by 2032, at a CAGR of 13.40% during the forecast period
- This growth is driven by factors such as rising prevalence of chronic, emergence of biosimilars and advancement in biotechnology
Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market Analysis
- Monoclonal antibody (mAb) therapeutics are pivotal in treating various diseases, including cancer, autoimmune disorders, and infectious diseases. Their specificity and efficacy have led to widespread adoption in modern medicine
- The demand for mAb therapies is significantly driven by the increasing prevalence of chronic diseases and advancements in biotechnology and genetic engineering. Techniques such as recombinant DNA technology and hybridoma methods have enhanced the development and production of monoclonal antibodies
- North America is expected to dominate the monoclonal antibody therapeutics market with market share of 45.7% due to advanced healthcare infrastructure, substantial healthcare expenditure, and strong regulatory support
- Asia-Pacific is expected to be the fastest growing region in the monoclonal antibody therapeutics market, with a market share of 30.6% during the forecast period. This growth is driven by rapid expansion in healthcare infrastructure, increasing awareness about chronic diseases, and rising demand for advanced therapeutic
- The cancer segment is expected to dominate the market with a market share of 50.5% in 2025, driven by the high prevalence of various cancers and the effectiveness of mAb therapies in targeting specific cancer cells such as those in breast cancer, lung cancer, and lymphoma
Report Scope and Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market Segmentation
|
Attributes |
Monoclonal Antibody Therapeutics Market / mABs Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market Trends
“Increasing Integration of Advanced Antibody Engineering and Precision Targeting Technologies”
- One prominent trend in the evolution of monoclonal antibody therapeutics is the increasing integration of advanced antibody engineering and precision targeting technologies
- These innovations enhance therapeutic efficacy by enabling antibodies to bind selectively to disease-specific antigens, reducing off-target effects and improving patient safety profiles
- For instance, next-generation mABs—such as bispecific antibodies and antibody-drug conjugates (ADCs)—are revolutionizing treatment by combining targeting precision with potent cytotoxic activity, particularly in oncology and hematological malignancies
- These advancements are transforming the landscape of disease treatment, expanding the range of treatable conditions, improving patient outcomes, and driving the demand for personalized, next-generation biologics in the global mAb therapeutics market
Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market Dynamics
Driver
“Rising Prevalence of Chronic and Autoimmune Diseases Fueling Demand”
- The increasing global burden of chronic conditions such as cancer, rheumatoid arthritis, multiple sclerosis, and Crohn’s disease is significantly driving the demand for monoclonal antibody therapeutics
- These diseases often require long-term treatment strategies, and monoclonal antibodies offer targeted, effective, and often better-tolerated alternatives to conventional therapies
- As awareness increases and more patients gain access to advanced biologics, the demand for mABs continues to rise across both developed and developing regions
For instance,
- In October 2023, according to a report by the World Health Organization (WHO), over 41 million people die each year from noncommunicable diseases, representing 74% of all global deaths, with cancer and autoimmune disorders being major contributors. The WHO emphasized the need for innovative therapies such as monoclonal antibodies to manage these rising health burdens
- As a result, pharmaceutical companies are investing heavily in research and development of next-generation monoclonal antibodies to meet the rising global need and address more disease targets
Opportunity
“Advancements in Antibody Engineering and Personalized Medicine”
- Innovations in antibody engineering, such as bispecific antibodies, antibody-drug conjugates (ADCs), and Fc-engineered mABs, are expanding the capabilities of mAb therapeutics across diverse therapeutic areas
- These advanced formats allow for enhanced specificity, improved immune system activation, and better delivery of cytotoxic agents directly to diseased cells, minimizing harm to healthy tissue
- The rise of personalized medicine and companion diagnostics further strengthens the potential of monoclonal antibodies by aligning treatments with individual patient profiles, improving outcomes, and reducing trial-and-error in treatment protocols
For instance,
- In January 2024, a study published in Nature Reviews Drug Discovery highlighted that over 180 monoclonal antibodies are currently in clinical trials, many of which incorporate targeted delivery and biomarker-based patient selection. These strategies represent a shift toward precision therapies that offer higher efficacy and lower toxicity
- The synergy of antibody engineering with genomics and data-driven drug design is expected to revolutionize treatment paradigms and unlock new growth avenues in the global mAb market
Restraint/Challenge
“High Manufacturing and Therapy Costs Limiting Accessibility”
- The complex manufacturing processes of monoclonal antibodies, including cell culture, purification, and quality control, contribute to very high production costs, which are often passed on to patients and healthcare systems
- These therapies can cost tens of thousands of dollars per year per patient, posing significant challenges for affordability and inclusion in public or private reimbursement schemes—especially in low- and middle-income countries
- Moreover, the need for cold-chain logistics, sterile facilities, and specialized healthcare professionals for administration further adds to the cost burden
For instance,
- According to a March 2024 report from the Journal of the American Medical Association (JAMA), the average annual treatment cost for monoclonal antibody therapies in the U.S. exceeds USD 100,000 per patient, making them among the most expensive drug classes. The report emphasized that such high prices may delay or prevent access to treatment for patients without comprehensive insurance coverage
- These barriers not only limit patient access to life-saving therapies but also slow market penetration in resource-constrained settings, posing a key challenge to market growth and equitable healthcare delivery
Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market Scope
The market is segmented on the basis of application, source and end user.
|
Segmentation |
Sub-Segmentation |
|
By Application |
|
|
By Source |
|
|
By End User |
|
In 2025, the cancer is projected to dominate the market with a largest share in application segment
The cancer segment is expected to dominate the monoclonal antibody therapeutics market / mABs therapeutics market with the largest share of 50.5% in 2025 due to its high prevalence and the effectiveness of mAb therapies in targeting specific cancer cells, such as in breast cancer, lung cancer, and lymphoma. As the leading therapeutic approach for cancer treatment, advancements in monoclonal antibody engineering and targeted therapies continue to improve patient outcomes, driving market growth. Increased cancer incidences globally further contribute to its market dominance.
The human segment is expected to account for the largest share during the forecast period in source market
In 2025, the human segment is expected to dominate the monoclonal antibody therapeutics market with the largest market share of 44.7% due to their lower immunogenicity and better patient tolerance, making them the preferred choice in therapeutic development. Advancements in genetic engineering have enabled the production of highly specific and low-immunogenic antibodies, improving therapeutic outcomes and driving market growth. The growing demand for more effective and targeted treatments for chronic diseases further contributes to its market dominance.
Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market Regional Analysis
“North America Holds the Largest Share in the Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market”
- North America dominates the monoclonal antibody therapeutics market, accounting for approximately 45.7% of the total market share. This dominance is driven by advanced healthcare infrastructure, high adoption of cutting-edge medical technologies, and the strong presence of key market players in the region
- U.S. holds a significant share of this, with approximately 73.8% of North America's market share, due to the increasing demand for high-precision therapeutics, the rising prevalence of chronic diseases such as cancer, rheumatoid arthritis, and autoimmune disorders, and continuous advancements in biotechnology and genetic engineering
- The availability of well-established reimbursement policies and growing investments in research & development by leading pharmaceutical and biotech companies further strengthen the market
- In addition, the growing adoption of biosimilars and personalized medicine, along with regulatory support, is fueling market expansion across North America
“Asia-Pacific is Projected to Register the Highest CAGR in the Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market”
- Asia-Pacific is expected to witness the highest growth rate in the monoclonal antibody therapeutics market, with a projected CAGR of 12.4% during the forecast period. This growth is driven by rapid expansion in healthcare infrastructure, increasing awareness about chronic diseases, and rising demand for advanced therapeutics
- The region is expected to hold approximately 30.6% of the global market share by 2025, with China, India, and Japan emerging as key markets
- Japan, with its advanced medical technology and increasing healthcare spending, remains a crucial market for monoclonal antibodies, accounting for 40% of the Asia-Pacific market share. Japan is at the forefront of adopting innovative biologics and targeted therapies to enhance patient outcomes
- India is projected to register the highest CAGR in the market, with a share of approximately 18.9% in the Asia-Pacific market, driven by expanding healthcare infrastructure, rising awareness about advanced treatments, and a growing patient base seeking access to monoclonal antibody therapies for various chronic conditions
Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd (Switzerland)
- Johnson & Johnson Services, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Amgen Inc. (U.S.)
- Sanofi (France)
- Novartis AG (Switzerland)
- AbbVie Inc. (U.S.)
- GSK plc. (U.K.)
- Regeneron Pharmaceuticals Inc. (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- AstraZeneca (U.K.)
- Takeda Pharmaceutical Company Limited (Japan)
- Bristol-Myers Squibb Company (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Gilead Sciences, Inc. (U.S)
- Lilly (U.S.)
Latest Developments in Global Monoclonal Antibody Therapeutics Market / mABs Therapeutics Market
- In January 2025, Roche announced the initiation of Phase III clinical trials for its novel monoclonal antibody RGX-202, targeting HER2-positive gastric cancer. This highlights Roche’s continued leadership in oncology biologics by expanding its pipeline to address unmet needs in gastric cancer treatment
- In February 2025, AbbVie received FDA approval for Skyrizi (risankizumab) for the treatment of moderate to severe Crohn’s disease. Skyrizi, a humanized monoclonal antibody, previously approved for psoriasis, represents AbbVie’s growing dominance in the autoimmune therapeutic segment
- In March 2025, the European Medicines Agency (EMA) approved Darzalex Faspro (daratumumab and hyaluronidase-fihj) by Johnson & Johnson for newly diagnosed multiple myeloma. This subcutaneous version offers increased convenience and reduced infusion time for patients
- In April 2025, Merck announced positive Phase III results from the KEYNOTE-522 trial for Keytruda (pembrolizumab) in early-stage triple-negative breast cancer, demonstrating improved response rates when combined with chemotherapy
- In May 2025, the European Commission approved Tezepelumab, developed by Amgen, for severe asthma. This monoclonal antibody targets thymic stromal lymphopoietin (TSLP), offering a new treatment option for patients unresponsive to standard therapies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.