Global Multi Cancer Early Detection Market
Market Size in USD Billion
CAGR :
% 
 USD
2.65 Billion
USD
8.13 Billion
2024
2032
USD
2.65 Billion
USD
8.13 Billion
2024
2032
| 2025 –2032 | |
| USD 2.65 Billion | |
| USD 8.13 Billion | |
|
|
|
|
Multi-Cancer Early Detection Market Size
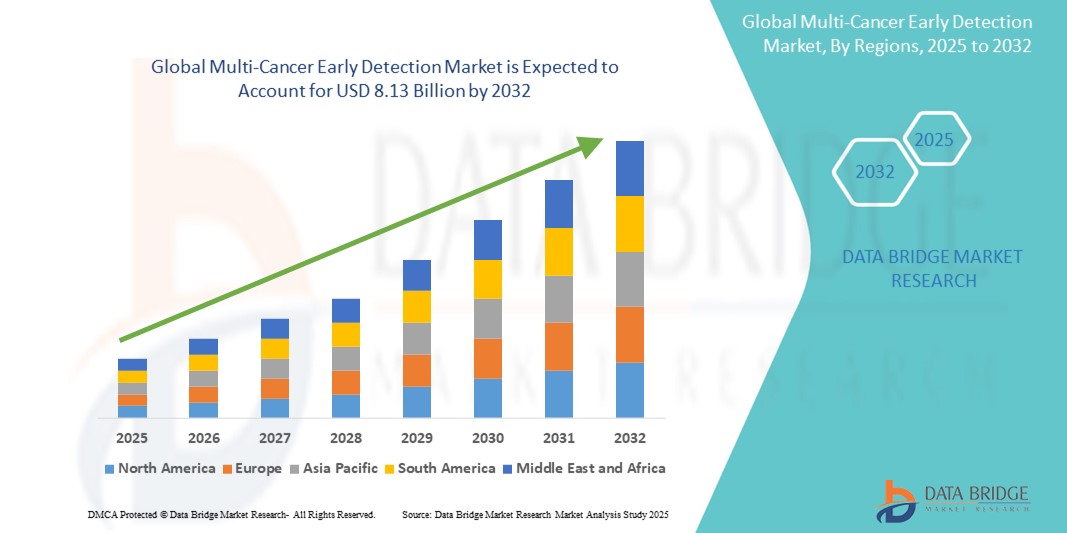
- The global multi-cancer early detection market size was valued at USD 2.65 billion in 2024 and is expected to reach USD 8.13 billion by 2032, at a CAGR of 15.02% during the forecast period
- The market growth is largely fueled by the increasing emphasis on early cancer diagnosis and the technological advancements in genomic and proteomic biomarkers, enabling broader applications of multi-cancer early detection (MCED) solutions across diverse populations
- Furthermore, rising demand for minimally invasive diagnostic techniques, particularly liquid biopsy-based MCED tests, is establishing these solutions as a transformative approach in oncology diagnostics. These converging factors are accelerating the adoption of Multi-Cancer Early Detection solutions, thereby significantly boosting the industry's growth
Multi-Cancer Early Detection Market Analysis
- The multi-cancer early detection (MCED) market is gaining momentum as a transformative approach in oncology, offering the ability to screen for multiple types of cancers through a single test, often using liquid biopsy techniques. This innovation is reshaping cancer diagnostics due to its non-invasive nature, early detection potential, and ability to improve survival rates
- The accelerating demand for MCED solutions is primarily driven by increasing cancer incidence rates globally, technological advancements in genomics and bioinformatics, and the growing emphasis on precision medicine and preventative healthcare
- North America dominated the multi-cancer early detection market with the largest revenue share of 42.3% in 2024, owing to robust R&D investments, favorable regulatory support, and widespread adoption of advanced diagnostic tools. The U.S., in particular, has witnessed significant uptake of MCED solutions, propelled by initiatives from biotech firms and healthcare providers integrating early detection tests into routine screenings
- Asia-Pacific is expected to be the fastest-growing region in the multi-cancer early detection market during the forecast period, with a projected CAGR of 21.3% from 2025 to 2032. This growth is fueled by increasing cancer burden, improving healthcare infrastructure, and rising awareness about early diagnostics, especially in countries like China, India, and Japan
- The Liquid Biopsy segment dominated the multi-cancer early detection market with the largest revenue share of 47.3% in 2024, owing to its non-invasive nature, ability to detect multiple cancers from a single blood draw, and growing preference among healthcare providers for early detection techniques
Report Scope and Multi-Cancer Early Detection Market Segmentation
|
Attributes |
Multi-Cancer Early Detection Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Multi-Cancer Early Detection Market Trends
“Growing Demand for Advanced and Integrated Screening Technologies”
- A significant and accelerating trend in the global multi-cancer early detection market is the integration of advanced analytics and personalized diagnostic platforms, which are reshaping the early detection landscape by offering increased precision and efficiency
- For instance, companies are leveraging machine learning algorithms and genomic profiling tools to enhance the accuracy of liquid biopsies and gene panels used in detecting multiple cancers from a single sample. These data-driven approaches enable the identification of cancer signals across various types with high sensitivity, even at early stages
- Many leading MCED platforms are also utilizing cloud-based diagnostics and centralized databases to improve clinical decision-making and streamline the patient workflow. These platforms assist clinicians in interpreting vast amounts of genomic and proteomic data, accelerating diagnosis and optimizing treatment pathways
- Furthermore, integration with electronic health records (EHRs) and digital health ecosystems allows healthcare providers to track patient outcomes and personalize screening protocols. Such interoperability also supports population-wide screening initiatives and early intervention strategies
- The convenience and reliability of at-home sample collection kits are also gaining momentum in the MCED market, empowering consumers to take proactive control of their health while supporting healthcare systems in reaching underserved or remote populations
- Companies like GRAIL, Guardant Health, and Exact Sciences are at the forefront, offering tests capable of identifying signals from multiple cancers before symptoms appear—drastically improving survival outcomes through earlier intervention
- This ongoing convergence of precision diagnostics, cloud computing, and patient-centric healthcare delivery is not only enhancing user engagement but also establishing multi-cancer early detection as a pivotal component in the global shift toward preventive oncology
Multi-Cancer Early Detection Market Dynamics
Driver
“Growing Need Due to Rising Cancer Incidence and Demand for Early Diagnosis”
- The increasing prevalence of cancer globally, coupled with the growing emphasis on early detection to improve patient outcomes and reduce healthcare costs, is a significant driver for the heightened demand for multi-cancer early detection (MCED) solutions
- For instance, in April 2024, GRAIL, LLC announced updates to its Galleri test, an MCED blood test capable of detecting more than 50 types of cancer from a single blood draw. The company aims to expand accessibility by partnering with health systems and employers. Such strategies by key companies are expected to drive the Multi-Cancer Early Detection industry growth during the forecast period
- As healthcare providers and patients become more aware of the benefits of early diagnosis, MCED solutions offer compelling advantages such as non-invasiveness, the ability to detect cancers before symptoms appear, and comprehensive genomic profiling from a single sample
- Furthermore, the growing availability of liquid biopsy and next-generation sequencing (NGS) platforms is making MCED tests a key component of personalized medicine initiatives, offering seamless integration with patient records and clinical workflows
- The convenience of at-home sample collection kits, digital test ordering, and rapid turnaround times are propelling the adoption of MCED technologies in both clinical and consumer health settings. The trend towards decentralized diagnostics and increasing availability of user-friendly Multi-Cancer Early Detection options further contribute to market growth
Restraint/Challenge
“Concerns Regarding False Positives and High Initial Testing Costs”
- Concerns surrounding the clinical validity and utility of some MCED tests—particularly the potential for false positives or inconclusive results—pose a significant challenge to broader market penetration. False positives may lead to unnecessary follow-up procedures, causing patient anxiety and increased costs
- For instance, health agencies have highlighted the need for clear regulatory pathways and rigorous clinical evidence to support the widespread adoption of MCED technologies
- Addressing these concerns through large-scale clinical validation, transparent performance metrics, and clear guidance for clinicians on interpreting test results is essential for building stakeholder trust. Companies like Exact Sciences and Guardant Health are investing in pivotal trials to showcase test accuracy, cancer site prediction, and long-term benefits
- In addition, the relatively high cost of some MCED solutions can be a barrier to adoption, especially in publicly funded healthcare systems or price-sensitive emerging markets. While costs are gradually decreasing, full insurance coverage and reimbursement remain limited in many regions
- Overcoming these challenges through broader clinical integration, payer engagement, and the development of more affordable and accessible MCED solutions will be vital for sustained market growth
Multi-Cancer Early Detection Market Scope
The market is segmented on the basis of type and end-use.
- By Type:
On the basis of type, the multi-cancer early detection market is segmented into liquid biopsy, gene panel, LDT (Laboratory Developed Tests), and others. The liquid biopsy segment dominated the market with the largest revenue share of 47.3% in 2024, owing to its non-invasive nature, ability to detect multiple cancers from a single blood draw, and growing preference among healthcare providers for early detection techniques.
The gene panel segment is expected to register the fastest CAGR of 23.9% from 2025 to 2032, driven by advancements in next-generation sequencing (NGS) and the rising demand for personalized oncology diagnostics. Gene panels offer high sensitivity in detecting specific cancer-associated mutations and are increasingly being integrated into screening workflows.
- By End-Use:
On the basis of end-use, the market is segmented into hospitals, diagnostic laboratories, and others. The hospitals segment accounted for the largest revenue share of 52.8% in 2024, attributed to the presence of advanced diagnostic infrastructure, strong physician-patient networks, and growing integration of MCED technologies into routine hospital screening programs.
The diagnostic laboratories segment is projected to grow at the highest CAGR of 22.1% from 2025 to 2032, driven by the increasing use of laboratory-developed MCED tests, centralized testing capabilities, and broader accessibility in urban and semi-urban settings.
Multi-Cancer Early Detection Market Regional Analysis
- North America dominated the multi-cancer early detection market with the largest revenue share of 42.3% in 2024, driven by the rapid adoption of advanced cancer screening tools, robust research infrastructure, and the presence of key biotech players. The region benefits from proactive government support, increased awareness programs, and well-established healthcare systems that support early cancer diagnosis
- Favorable reimbursement policies, high healthcare spending, and rising cancer prevalence further accelerate the demand for early detection technologies across the U.S. and Canada
- Continuous innovation in liquid biopsy and genomic testing also contributes to the region’s market dominance, particularly in personalized cancer diagnostics
U.S. Multi-Cancer Early Detection Market Insight
The U.S. multi-cancer early detection market captured the largest revenue share of 71% in 2024 within North America, driven by rising investments in oncology diagnostics and early detection R&D. The widespread availability of genomic sequencing, adoption of liquid biopsy, and the emergence of AI-powered diagnostic tools continue to boost market growth. Growing public-private partnerships and initiatives from organizations like the National Cancer Institute (NCI) are further accelerating the deployment of multi-cancer early detection tests across hospitals and diagnostic labs.
Europe Multi-Cancer Early Detection Market Insight
The Europe multi-cancer early detection market is projected to expand at a substantial CAGR during the forecast period, fueled by increasing awareness of cancer prevention, early diagnosis initiatives, and strong regulatory support for innovative diagnostics. European governments are supporting precision medicine programs and cancer screening campaigns, which are improving accessibility and adoption of advanced multi-cancer detection tools. The growing number of clinical collaborations and research consortia across countries is fostering a favorable environment for market growth.
U.K. Multi-Cancer Early Detection Market Insight
The U.K. multi-cancer early detection market is anticipated to grow at a noteworthy CAGR, driven by government-backed screening programs such as the NHS-Galleri trial and national initiatives focused on early cancer intervention. The demand is further enhanced by a tech-savvy population and the expansion of centralized laboratory infrastructure. The U.K.’s robust focus on early diagnostics, personalized medicine, and integration of genomic data into clinical workflows is playing a pivotal role in shaping the market.
Germany Multi-Cancer Early Detection Market Insight
The Germany multi-cancer early detection market is expected to expand at a considerable CAGR, supported by high awareness levels, a strong healthcare system, and increased funding in oncology research. The country’s emphasis on integrating digital health solutions with diagnostic services is paving the way for multi-cancer early detection tests. Germany's widespread acceptance of genetic testing and personalized care, especially in university hospitals and cancer centers, also fuels market momentum.
Asia-Pacific Multi-Cancer Early Detection Market Insight
The Asia-Pacific multi-cancer early detection market is poised to grow at the fastest CAGR of 21.3% during the forecast period of 2025 to 2032, attributed to growing cancer incidence, increasing healthcare investments, and improving diagnostic infrastructure across emerging economies. Countries like China, Japan, and India are witnessing surging demand for non-invasive, cost-effective cancer detection solutions, particularly in urban regions. Government-led cancer control programs and expanding biotechnology sectors are expected to further boost market uptake.
Japan Multi-Cancer Early Detection Market Insight
The Japan multi-cancer early detection market is gaining traction, driven by rapid aging of the population, high incidence of cancer, and strong focus on preventive healthcare. Japan’s national screening strategies and high-tech medical ecosystem support the adoption of advanced diagnostic platforms, including multi-cancer early detection tests. Local innovations, academic research partnerships, and integration of AI in pathology also contribute to growth.
China Multi-Cancer Early Detection Market Insight
The China multi-cancer early detection market held the largest revenue share in Asia-Pacific in 2024, due to a rising middle-class population, government efforts in cancer control, and booming demand for personalized medicine. The growing use of liquid biopsy, rapid expansion of healthcare infrastructure, and increased funding in biotech startups developing early cancer detection platforms are key growth drivers. Moreover, the push for “Healthy China 2030” strengthens early diagnosis as a national healthcare priority.
Multi-Cancer Early Detection Market Share
The multi-cancer early detection industry is primarily led by well-established companies, including:
- Micronoma, Inc. (U.S.)
- ANPAC Bio-Medical Science Co., Ltd (U.S.)
- EARLYDIAGNOSTICS (U.S.)
- Early is Good (U.S.)
- CanSense Ltd (U.S.)
- Freenome Holdings, Inc. (U.S.)
- Oncocyte Corporation (U.S.)
- SeekIn, Inc. (U.S.)
- Naveris (U.S.)
- VESEN, Inc. (U.S.)
- Grail, LLC (Illumina, Inc.) (U.S.)
- Exact Sciences Corporation (U.S.)
- FOUNDATION MEDICINE, INC. (U.S.)
- Guangzhou AnchorDx Medical Co., Ltd. (China)
- Guardant Health (U.S.)
- Burning Rock Biotech Limited (China)
- Genecast Biotechnology Co., Ltd (China)
- Singlera Genomics, Inc. (U.S.)
Latest Developments in Global Multi-Cancer Early Detection Market
- In July 2025, the U.S. National Cancer Institute (NCI) launched the Vanguard Study, enrolling 24,000 adults aged 45–70 to test the effectiveness of multi-cancer early detection (MCED) blood tests by GRAIL and ClearNote Health. The study aims to compare the ability of these tests to detect multiple cancers against standard screening. This large-scale initiative represents a pivotal step toward regulatory approval and widespread clinical use of MCED tests
- In June 2025, GRAIL Inc. announced positive top-line results from its pivotal PATHFINDER 2 study of the Galleri test, covering 25,578 participants. The trial demonstrated strong performance with a positive predictive value (PPV) of 43%, specificity of 99.5%, and a cancer signal origin prediction accuracy of 91%. These findings provide critical clinical validation for Galleri as an effective MCED solution
- In May 2025, Guardant Health received FDA Breakthrough Device Designation for its Shield MCED test, which uses a proprietary methylation-based approach to detect early-stage cancers through a single blood draw. In clinical studies, the test demonstrated a specificity of 98.6% and sensitivity of 75% across several cancer types, enabling faster regulatory pathways for broader access
- In April 2025, GRAIL and athenahealth announced a partnership to integrate Galleri test ordering directly into athenaOne, a leading cloud-based EHR platform. This integration simplifies clinician workflows by enabling over 160,000 providers to order MCED tests seamlessly from their existing systems, accelerating clinical adoption
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.