Global Nanomedicine In Central Nervous System Injury And Repair Market
Market Size in USD Billion
CAGR :
% 
 USD
38.72 Billion
USD
82.45 Billion
2025
2033
USD
38.72 Billion
USD
82.45 Billion
2025
2033
| 2026 –2033 | |
| USD 38.72 Billion | |
| USD 82.45 Billion | |
|
|
|
|
Nanomedicine in Central Nervous System Injury and Repair Market Size
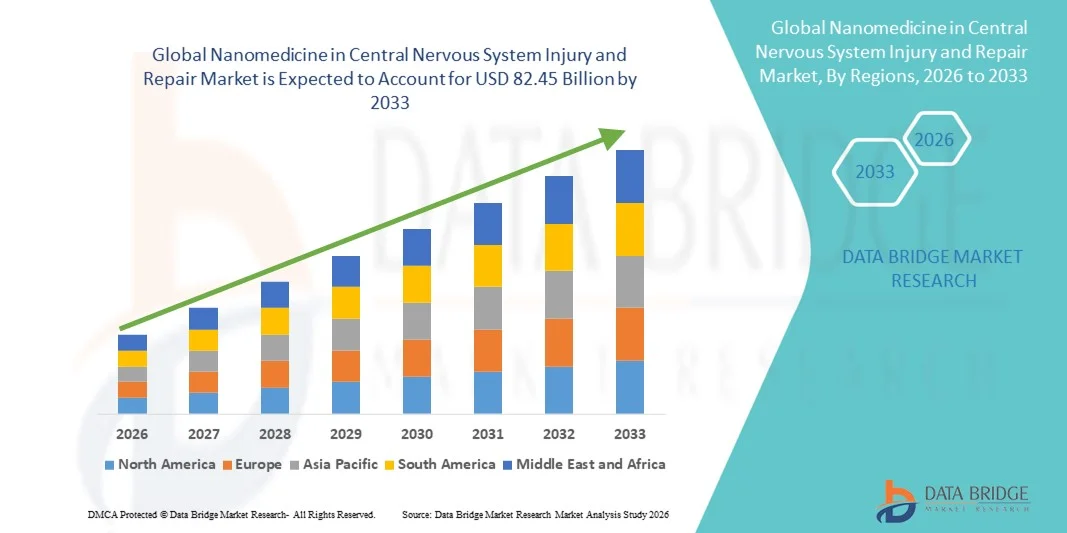
- The global nanomedicine in central nervous system injury and repair market size was valued at USD 38.72 billion in 2025 and is expected to reach USD 82.45 billion by 2033, at a CAGR of 9.91% during the forecast period
- The market expansion is largely fueled by advancements in nanomedicine for targeted drug delivery, regenerative therapies, and nanostructured tools that can cross the blood‑brain barrier and interact at the cellular and molecular level to treat neuronal injury and neurodegenerative conditions
- Furthermore, growing investments in R&D, rising awareness of nanomedicine’s potential in CNS repair, and heightened government and private funding for life‑science innovations are driving the adoption of these solutions in clinical and research settings
Nanomedicine in Central Nervous System Injury and Repair Market Analysis
- Nanomedicine in central nervous system injury and repair market, leveraging therapeutics, regenerative medicine, and advanced diagnostics, is increasingly vital in addressing neurological disorders, spinal cord injuries, and neurodegenerative conditions due to its ability to cross the blood-brain barrier, target damaged neural tissue, and promote repair at the cellular and molecular levels
- The escalating demand for CNS-targeted nanomedicine is primarily fueled by the rising prevalence of CNS injuries, growing incidence of neurodegenerative diseases, and increasing awareness of the limitations of conventional therapies, which drives adoption of more precise and effective nanotherapeutic and regenerative approaches
- North America dominated the nanomedicine in central nervous system injury and repair market with the largest revenue share of 42.6% in 2025, characterized by advanced healthcare infrastructure, high R&D investments, and a strong presence of key industry players, with the U.S. leading in clinical trials and commercialization of CNS repair nanomedicines driven by innovations in targeted therapeutics and regenerative therapies
- Asia-Pacific is expected to be the fastest-growing region in the nanomedicine in central nervous system injury and repair market during the forecast period due to increasing healthcare investments, rising prevalence of neurological disorders, and expanding collaborations between local biotech firms and global nanomedicine developers
- The therapeutics segment dominated the nanomedicine in central nervous system injury and repair market with a market share of 48.4% in 2025, driven by its central role in repairing neural damage, improving functional recovery, and providing targeted treatment solutions, while regenerative medicine and diagnostic applications are emerging as key growth areas
Report Scope and Nanomedicine in Central Nervous System Injury and Repair Market Segmentation
|
Attributes |
Nanomedicine in Central Nervous System Injury and Repair Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Nanomedicine in Central Nervous System Injury and Repair Market Trends
Targeted Drug Delivery and Regenerative Therapies
- A significant and accelerating trend in the nanomedicine in central nervous system injury and repair market is the increasing use of nanoparticle-based targeted drug delivery systems and regenerative medicine approaches, enabling precise treatment of CNS injuries and neurodegenerative disorders
- For instance, lipid-based nanoparticles are being utilized to deliver neuroprotective agents directly to injured neurons, improving efficacy while minimizing systemic side effects. Similarly, stem-cell loaded nanocarriers are being explored for spinal cord and brain tissue regeneration
- Advancements in nanomedicine allow for enhanced bioavailability, controlled release, and improved penetration across the blood-brain barrier, which substantially improves therapeutic outcomes and reduces off-target effects. For instance, polymeric nanocarriers have shown improved drug retention in CNS tissues, enhancing functional recovery in preclinical studies
- Integration of diagnostics with therapeutics (theranostics) is enabling personalized treatment monitoring, allowing clinicians to track repair progress and adjust therapy in real-time, creating a more precise and adaptive CNS treatment paradigm
- This trend towards highly targeted, multifunctional nanomedicine solutions is driving R&D investments and encouraging biotech companies such as Bioasis and Nanobiotix to develop combined therapeutic and diagnostic platforms
- The demand for CNS-targeted nanomedicine is growing rapidly across clinical and research applications, as healthcare providers and researchers increasingly prioritize precision, efficacy, and safety in CNS injury repair
- Integration of AI and machine learning for nanomedicine formulation optimization is enhancing drug targeting accuracy and accelerating preclinical testing timelines
Nanomedicine in Central Nervous System Injury and Repair Market Dynamics
Driver
Rising Prevalence of CNS Disorders and Unmet Clinical Needs
- The increasing incidence of CNS injuries, neurodegenerative diseases, and traumatic brain or spinal cord injuries is a significant driver for the growing adoption of nanomedicine in CNS repair
- For instance, in 2025, Nanobiotix announced preclinical success of a nanoparticle-based therapy for targeted neuroprotection, demonstrating accelerated neuronal recovery in animal models. Such breakthroughs by key players are expected to propel market growth in the forecast period
- As conventional therapies struggle to effectively cross the blood-brain barrier, nanomedicine offers advanced delivery platforms, regenerative therapies, and diagnostic tools that address critical treatment gaps
- Furthermore, increasing investments by public and private institutions into CNS research and nanotechnology are driving rapid development and adoption of innovative therapeutics and regenerative solutions
- Personalized and targeted nanomedicine approaches, including theranostics and biomaterial-based regenerative therapies, are enhancing clinical outcomes and driving demand across both hospital and research settings
- The ability to reduce systemic side effects while improving recovery and functional outcomes is making CNS-targeted nanomedicine an increasingly preferred approach for clinicians and researchers
- For instance, collaborations between nanomedicine developers and hospitals are accelerating translational research and clinical adoption of CNS repair therapies
- Growing awareness of long-term disability prevention and quality-of-life improvement in patients with CNS injuries is boosting demand for nanomedicine-based interventions
Restraint/Challenge
Regulatory Hurdles and High Development Costs
- Stringent regulatory requirements for nanomedicine safety, efficacy, and quality, coupled with the complexity of CNS-targeted therapies, pose significant challenges to broader market adoption
- For instance, delays in clinical approvals due to lack of standardized nanotoxicology protocols have slowed the commercialization of several promising nanoparticle-based CNS therapies
- High research and development costs, specialized manufacturing requirements, and the need for extensive preclinical and clinical testing increase the financial and operational burden for companies entering this market
- While some innovative nanomedicine platforms have received accelerated regulatory support, the overall cost and complexity can deter smaller biotech firms from developing CNS-targeted therapies
- Overcoming these challenges requires enhanced regulatory guidance, standardized evaluation methods, and cost-optimization strategies in production and clinical development
- Companies that can balance safety, efficacy, and affordability while navigating regulatory landscapes are best positioned for sustained growth in the nanomedicine in central nervous system injury and repair market
- For instance, lack of harmonized international regulations on nanomedicine manufacturing and quality standards can delay global market entry
- High uncertainty in long-term clinical outcomes and limited real-world efficacy data may hinder physician adoption and investor confidence in new CNS nanomedicine therapies
Nanomedicine in Central Nervous System Injury and Repair Market Scope
The market is segmented on the basis of product and application.
- By Product
On the basis of product, the nanomedicine in central nervous system injury and repair market is segmented into therapeutics, regenerative medicine, in-vitro diagnostics, in-vivo diagnostics, and vaccines. The Therapeutics segment dominated the market with the largest market revenue share of 48.4% in 2025, driven by its critical role in repairing neural tissue and managing neurodegenerative disorders. Nanoparticle-based therapeutics enable targeted drug delivery across the blood-brain barrier, enhancing drug bioavailability while reducing systemic side effects. Therapies include neuroprotective agents, anti-inflammatory drugs, and neurotrophic factors that accelerate functional recovery after CNS injury. Healthcare providers increasingly prefer therapeutics due to their direct impact on clinical outcomes and the growing body of clinical evidence supporting efficacy. Furthermore, advancements in controlled-release formulations and biodegradable nanocarriers are expanding the applicability of therapeutic nanomedicine. The segment’s dominance is reinforced by strong ongoing investments from biotech firms and research institutions focused on novel CNS-targeted therapies.
The Regenerative Medicine segment is anticipated to witness the fastest growth rate of 21% CAGR from 2026 to 2033, fueled by increasing applications in spinal cord injury and traumatic brain repair. Regenerative approaches use stem-cell loaded nanoparticles and tissue-engineered scaffolds to promote neural regeneration, offering potential long-term recovery where conventional therapies fail. Rising prevalence of CNS injuries and neurodegenerative conditions globally is accelerating adoption in both clinical and research settings. Regenerative medicine also benefits from advances in biomaterials, gene therapy integration, and nanocarrier design, allowing precise targeting of damaged neural regions. The growing investment by governments and private players to develop regenerative nanomedicine solutions further drives market growth. In addition, regenerative medicine addresses unmet clinical needs, enhancing the quality of life for patients with otherwise limited treatment options.
- By Application
On the basis of application, the market is segmented into clinical oncology, infectious diseases, clinical cardiology, orthopedics, and others. The Clinical Oncology segment dominated the nanomedicine in central nervous system injury and repair market in 2025, accounting for 40% of the revenue, due to the rising incidence of brain tumors and CNS malignancies that require precision-targeted therapies. Nanomedicine enables highly localized delivery of chemotherapeutics and radiosensitizers, minimizing damage to surrounding healthy neural tissue. In addition, the integration of diagnostic imaging agents with therapeutic nanoparticles (theranostics) allows real-time monitoring of treatment efficacy. The presence of multiple ongoing clinical trials and FDA-supported programs for CNS oncology nanomedicine is reinforcing the segment’s market share. Strong partnerships between biotech firms and hospitals are facilitating faster adoption and commercialization. The need for improved survival rates and patient outcomes in CNS oncology continues to sustain segment dominance.
The Infectious Diseases segment is expected to witness the fastest CAGR of 22% from 2026 to 2033, driven by increasing research into nanomedicine-based antiviral and antibacterial therapies for CNS infections such as meningitis and viral encephalitis. Nanocarriers allow targeted delivery of antimicrobial agents directly to the CNS, overcoming the limitations of conventional systemic treatments. Growing awareness of CNS infection risks and the limitations of standard therapies is driving adoption in hospitals and research centers. In addition, the rising prevalence of antibiotic-resistant pathogens is boosting demand for nanomedicine innovations. Funding from public health organizations and collaborations with biotech startups are accelerating the development of novel CNS-targeted infectious disease treatments. The segment’s growth is further supported by advances in nanotechnology-enabled diagnostics that improve early detection and treatment monitoring.
Nanomedicine in Central Nervous System Injury and Repair Market Regional Analysis
- North America dominated the nanomedicine in central nervous system injury and repair market with the largest revenue share of 42.6% in 2025, characterized by advanced healthcare infrastructure, high R&D investments, and a strong presence of key industry players
- Researchers and healthcare providers in the region highly value the precision, targeted delivery, and enhanced therapeutic outcomes offered by nanomedicine solutions, which enable effective CNS repair and neuroregeneration with minimal systemic side effects
- This widespread adoption is further supported by advanced clinical trial networks, supportive government funding, and the presence of leading biotech companies developing innovative CNS-targeted therapeutics, regenerative medicine solutions, and nanodiagnostics, establishing North America as a key hub for both research and commercialization in this market
U.S. Nanomedicine in Central Nervous System Injury and Repair Market Insight
The U.S. market captured the largest revenue share of 80% in North America in 2025, fueled by the strong presence of leading biotech firms and advanced clinical research infrastructure. Researchers and hospitals increasingly prioritize CNS-targeted nanomedicine for its precision, safety, and ability to repair neural tissue. The growing trend of personalized medicine and rising prevalence of neurodegenerative disorders are further propelling adoption. Government funding and supportive regulatory frameworks accelerate the development and commercialization of nanoparticle-based therapeutics and regenerative solutions. In addition, the integration of diagnostic nanotechnologies with therapeutics is enhancing treatment monitoring and clinical outcomes, reinforcing U.S. market dominance.
Europe Nanomedicine in Central Nervous System Injury and Repair Market Insight
The Europe market is projected to expand at a substantial CAGR throughout the forecast period, driven by the rising incidence of CNS injuries and neurodegenerative diseases across aging populations. The demand for precision-targeted nanotherapeutics and regenerative medicine is growing in both hospital and research settings. Increased healthcare expenditure, well-established clinical trial networks, and rising adoption of advanced diagnostics further support market expansion. European researchers and clinicians favor nanomedicine for its ability to enhance functional recovery and reduce systemic side effects. Collaborations between regional biotech companies and global nanomedicine developers are fostering innovation. Regulatory encouragement for novel CNS therapies is also accelerating adoption across the continent.
U.K. Nanomedicine in Central Nervous System Injury and Repair Market Insight
The U.K. market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increased focus on neurodegenerative disease management and CNS injury repair. Rising awareness of novel therapeutic options and growing investment in clinical trials encourage adoption of nanoparticle-based therapies and regenerative medicine. Hospitals and research institutes are integrating nanomedicine solutions to improve patient outcomes and enable personalized treatment plans. The U.K.’s robust biotechnology ecosystem and e-health infrastructure facilitate rapid commercialization. In addition, the increasing prevalence of CNS disorders, coupled with strong government support for innovation, reinforces market growth.
Germany Nanomedicine in Central Nervous System Injury and Repair Market Insight
The Germany market is expected to expand at a considerable CAGR during the forecast period, fueled by rising demand for advanced nanomedicine solutions to treat CNS injuries and neurodegenerative conditions. The country’s well-developed healthcare infrastructure, research-oriented ecosystem, and emphasis on precision medicine promote adoption. Hospitals increasingly incorporate targeted therapeutics and regenerative therapies for enhanced functional recovery. German regulatory frameworks supporting innovative nanomedicine platforms also contribute to market growth. Patient awareness of CNS treatment options and emphasis on clinical efficacy further encourage adoption of these technologies. Integration of diagnostics with therapeutics is gaining traction, supporting comprehensive treatment approaches.
Asia-Pacific Nanomedicine in Central Nervous System Injury and Repair Market Insight
The Asia-Pacific market is poised to grow at the fastest CAGR of 24% from 2026 to 2033, driven by rising CNS disorder prevalence, expanding healthcare infrastructure, and government initiatives promoting advanced therapeutics. Countries such as China, Japan, and India are witnessing growing investment in clinical trials and nanomedicine R&D. Increased awareness of neurodegenerative disease management and CNS injury treatment is fostering adoption in hospitals and research institutions. Furthermore, collaborations with global biotech firms are accelerating technology transfer and localized production. The affordability of emerging nanomedicine solutions and improving regulatory support are making these therapies accessible to a broader patient population.
Japan Nanomedicine in Central Nervous System Injury and Repair Market Insight
The Japan market is gaining momentum due to rapid urbanization, technological advancement, and a high-tech healthcare ecosystem. Rising prevalence of CNS disorders among the aging population is driving demand for precise nanomedicine therapeutics and regenerative solutions. Integration of nanomedicine with diagnostic platforms allows real-time monitoring and improved treatment personalization. Hospitals and research institutions prioritize nanoparticle-based drug delivery for enhanced efficacy and minimal side effects. Government-backed innovation programs and strong clinical trial infrastructure accelerate commercialization. In addition, patient awareness and acceptance of advanced therapies continue to drive market expansion.
India Nanomedicine in Central Nervous System Injury and Repair Market Insight
The India market accounted for the largest revenue share in Asia-Pacific in 2025, attributed to rapid urbanization, rising CNS disorder incidence, and increasing technological adoption in healthcare. Hospitals and research centers are increasingly implementing nanomedicine therapeutics and regenerative medicine solutions. Government initiatives supporting healthcare innovation and clinical research infrastructure boost market growth. Collaboration with global biotech firms and domestic manufacturers ensures localized production and affordability. Rising patient awareness of advanced CNS treatments and the expansion of hospital networks further stimulate adoption. The focus on accessible, cost-effective nanomedicine solutions is establishing India as a key regional hub for CNS injury and repair therapies.
Nanomedicine in Central Nervous System Injury and Repair Market Share
The Nanomedicine in Central Nervous System Injury and Repair industry is primarily led by well-established companies, including:
- Abbott (U.S.)
- Pfizer Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Merck & Co., Inc. (U.S.)
- Alnylam Pharmaceuticals (U.S.)
- Biodexa Pharmaceuticals (U.S.)
- AxoNeural Therapeutics, Inc. (U.S.)
- Nanocarry (U.S.)
- Kannalife Sciences Inc. (U.S.)
- Nanobiotix (France)
- MagForce AG (Germany)
- Denali Therapeutics (U.S.)
- F. Hoffmann La Roche AG (Switzerland)
- Biogen Inc. (U.S.)
- AviadoBio (U.K.)
- Kriya Therapeutics (U.S.)
- Arrowhead Pharmaceuticals, Inc. (U.S.)
- CytImmune Sciences (U.S.)
- InanoBio (U.S.)
What are the Recent Developments in Global Nanomedicine in Central Nervous System Injury and Repair Market?
- In October 2025, a study published highlighted theranostic nanomaterials capable of simultaneously delivering drugs and monitoring injury response in traumatic brain injury (TBI), showing advances in nanomedicine platforms that combine targeted therapeutic delivery and real‑time biological sensing within the CNS
- In August 2025, researchers at the Institute of Process Engineering (Chinese Academy of Sciences) developed an exosome‑based therapeutic agent (SeNExo) for treating traumatic CNS injuries, which alleviated neuronal cell death, restored glial homeostasis, and improved locomotor recovery in mouse models of traumatic brain injury (TBI) and spinal cord injury (SCI), demonstrating potent therapeutic efficacy of nano‑enabled biologics
- In April 2025, scientists reported a major breakthrough in using peptide‑functionalized polymeric nanoparticles to deliver anti‑inflammatory agents across the blood‑brain barrier into specific brain regions, significantly improving physiological outcomes in preclinical models and opening new avenues for treating neurological conditions such as Alzheimer’s and multiple sclerosis with targeted nanomedicine
- In February 2025, BioArctic, a Swedish biotech known for its Alzheimer’s therapy technology, announced it was seeking partners to expand its blood‑brain barrier (BBB) transporter technology a biochemical method that mimics natural transport mechanisms to deliver therapeutic antibodies into the brain, which could significantly enhance delivery of nanomedicine agents for a range of CNS disorders
- In February 2024, researchers at the Polytechnic University of Milan demonstrated a nanogel‑based nano‑vector capable of delivering anti‑inflammatory drugs directly into glial cells involved in spinal cord injury (SCI), offering a novel targeted approach to reduce inflammation and promote neural repair in SCI models potentially improving functional recovery where existing treatments fall short
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.