Global Neonatal Intensive Care Market
Market Size in USD Billion
CAGR :
% 
 USD
8.26 Billion
USD
12.22 Billion
2025
2033
USD
8.26 Billion
USD
12.22 Billion
2025
2033
| 2026 –2033 | |
| USD 8.26 Billion | |
| USD 12.22 Billion | |
|
|
|
|
Neonatal Intensive Care Market Size
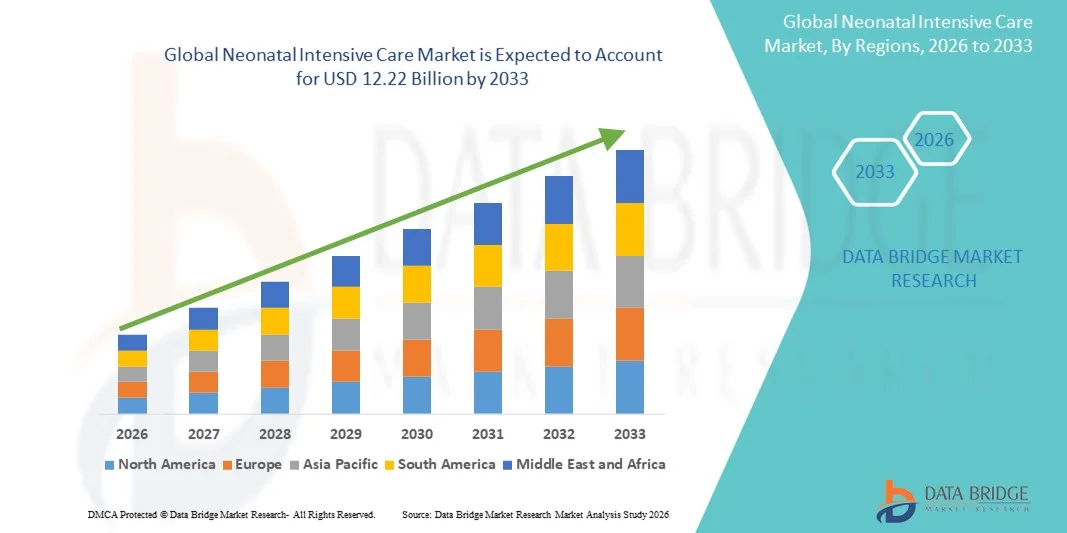
- The global neonatal intensive care market size was valued at USD 8.26 billion in 2025 and is expected to reach USD 12.22 billion by 2033, at a CAGR of 5.02% during the forecast period
- The market growth is primarily driven by the increasing prevalence of preterm births, neonatal complications, and advancements in neonatal care technologies, including ventilators, incubators, and monitoring systems. This is complemented by rising healthcare investments and the expansion of specialized neonatal care units across hospitals and clinics
- In addition, growing awareness among parents and healthcare providers about early intervention and neonatal survival, along with supportive government initiatives, is fueling the adoption of advanced neonatal intensive care solutions. These factors collectively are accelerating market demand, thereby significantly boosting the industry's growth
Neonatal Intensive Care Market Analysis
- Neonatal intensive care units (NICUs), providing specialized medical care for critically ill and premature newborns, are increasingly essential components of modern hospitals and healthcare facilities due to their life-saving interventions, advanced monitoring systems, and integration with pediatric and maternal care services
- The rising demand for NICU services is primarily driven by increasing preterm births, neonatal complications, and growing awareness among parents and healthcare providers regarding early intervention and neonatal survival
- North America dominated the neonatal intensive care market with the largest revenue share of 38.9% in 2025, characterized by advanced healthcare infrastructure, high healthcare spending, and a strong presence of key NICU equipment manufacturers, with the U.S. witnessing significant growth in NICU bed installations and adoption of cutting-edge monitoring and respiratory devices
- Asia-Pacific is expected to be the fastest-growing region in the neonatal intensive care market during the forecast period due to improving healthcare infrastructure, rising government initiatives for maternal and child health, and increasing hospital expansions in urban centers
- Respiratory devices segment dominated the neonatal intensive care market with a market share of 40.3% in 2025, driven by their essential role in supporting neonatal breathing, managing respiratory distress, and ensuring survival and proper development of critically ill newborns
Report Scope and Neonatal Intensive Care Market Segmentation
|
Attributes |
Neonatal Intensive Care Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Neonatal Intensive Care Market Trends
Advancements Through AI-Enabled Monitoring and Telehealth Integration
- A significant and accelerating trend in the global neonatal intensive care market is the integration of artificial intelligence (AI) and telehealth platforms into NICU monitoring systems, enhancing predictive care and continuous remote monitoring of neonates
- For instance, AI-enabled monitoring devices can track vital signs, detect early signs of distress, and alert clinicians instantly, allowing timely intervention and reducing neonatal mortality rates. Similarly, telehealth platforms enable pediatric specialists to remotely guide NICU care in smaller or rural hospitals
- AI integration in NICU equipment enables predictive analytics for complications, personalized ventilator settings, and intelligent alerts for unusual physiological patterns. For instance, some neonatal monitoring systems utilize AI to predict apnea events and optimize oxygen therapy based on real-time data
- The seamless integration of NICU devices with hospital information systems and telehealth networks facilitates centralized monitoring, data collection, and remote consultations, ensuring coordinated and efficient care across multiple departments
- This trend towards more intelligent, connected, and data-driven neonatal care is fundamentally reshaping expectations for NICU standards. Consequently, companies such as Draeger and GE Healthcare are developing AI-enabled neonatal monitors with predictive alert systems and telehealth compatibility
- The demand for advanced AI and telehealth-enabled neonatal care solutions is growing rapidly across hospitals and specialized care centers, as healthcare providers increasingly prioritize early intervention, continuous monitoring, and improved neonatal outcomes
- Emerging integration of wearable neonatal sensors is enabling continuous non-invasive monitoring of newborns’ vital signs, improving patient comfort and reducing the risk of infections from frequent handling
- Growing adoption of cloud-based NICU management platforms is allowing hospitals to store, analyze, and share patient data securely, enhancing clinical decision-making and facilitating multicenter neonatal research collaborations
Neonatal Intensive Care Market Dynamics
Driver
Increasing Preterm Births and Rising Neonatal Complications
- The increasing prevalence of preterm births and neonatal health complications, combined with growing awareness about early intervention, is a significant driver for the rising demand for advanced NICU solutions
- For instance, in March 2025, GE Healthcare launched upgraded neonatal ventilators with integrated monitoring systems to support premature infants with respiratory distress, driving adoption in leading hospitals
- As parents and healthcare providers seek improved survival and care outcomes for critically ill newborns, NICU equipment with continuous monitoring, predictive analytics, and integrated care solutions is increasingly preferred over conventional methods
- Furthermore, the expansion of hospital neonatal units and growing investments in healthcare infrastructure are making advanced NICU devices an essential component of pediatric care, ensuring safer and more effective treatment
- The ability to provide real-time monitoring, precise medication administration, and remote consultations is key to driving adoption of advanced NICU equipment in hospitals, clinics, and specialized neonatal care centers
- Increasing government initiatives and funding programs targeting maternal and child health are further encouraging hospitals to adopt advanced NICU technologies to reduce neonatal mortality rates
- Rising awareness campaigns among parents and healthcare providers about early detection of neonatal complications are creating higher demand for innovative NICU solutions that offer predictive and preventative care
Restraint/Challenge
High Costs and Regulatory Compliance Hurdles
- The high cost of advanced NICU equipment and the complex regulatory landscape for medical devices pose significant challenges to market expansion, particularly in developing regions with limited healthcare budgets
- For instance, reports of costly AI-enabled incubators and monitoring systems have made some smaller hospitals hesitant to adopt the latest NICU technologies despite their clinical benefits
- Addressing these cost barriers through leasing models, government subsidies, and gradual adoption strategies is crucial for broader penetration, while adherence to stringent regulatory standards ensures safety and efficacy of neonatal care devices
- In addition, concerns regarding maintenance, staff training, and technical support for sophisticated NICU systems can hinder adoption in hospitals with limited resources or expertise
- Overcoming these challenges through cost-effective solutions, simplified device interfaces, and compliance with regulatory guidelines will be vital for sustained growth and wider accessibility of neonatal intensive care services
- Limited availability of trained neonatal healthcare professionals, especially in rural or underdeveloped regions, poses a challenge to fully utilizing advanced NICU technologies
- Compatibility issues between legacy hospital systems and new NICU devices can impede seamless integration, delaying adoption and affecting operational efficiency
Neonatal Intensive Care Market Scope
The market is segmented on the basis of type, product, application, and end user
- By Type
On the basis of type, the neonatal intensive care market is segmented into peripheral catheters, central catheters, introducers, and accessories. The central catheters segment dominated the market with the largest revenue share in 2025, driven by their critical role in administering fluids, medications, and parenteral nutrition to critically ill neonates. Hospitals and NICUs often prioritize central catheters due to their reliability, precision, and ability to deliver essential treatments over extended periods. Central catheters also reduce the need for repeated needle sticks, thereby minimizing neonatal discomfort and risk of infection. The segment benefits from technological innovations such as ultrasound-guided insertion and antimicrobial coatings, improving safety and patient outcomes. Increasing adoption in high-volume NICUs and expanding neonatal care infrastructure in developed regions further strengthens its market dominance.
The peripheral catheters segment is anticipated to witness the fastest growth from 2026 to 2033, fueled by their ease of insertion and cost-effectiveness. Peripheral catheters are widely used for short-term medication administration and IV fluid delivery in neonates, making them suitable for both hospitals and smaller clinics. Their growing adoption is also supported by advancements in flexible and low-risk catheter materials that reduce complications such as infiltration or thrombosis. Peripheral catheters are increasingly preferred in emerging regions where NICU capacity is expanding rapidly but budgets are constrained. In addition, their compatibility with modern monitoring devices and integration into multi-lumen setups contributes to their rising demand.
- By Product
On the basis of product, the neonatal intensive care market is segmented into infant warmers, incubators, respiratory devices, neonatal monitoring devices, convertible warmer and incubators, phototherapy equipment, catheters, and others. The respiratory devices segment dominated the market in 2025 with a share of 40.3%, owing to the high incidence of neonatal respiratory distress syndrome and other pulmonary complications. These devices, including ventilators and CPAP systems, are essential for maintaining oxygenation and supporting breathing in premature and critically ill neonates. Hospitals prioritize respiratory devices for their life-saving capabilities, integration with monitoring systems, and the ability to provide precise respiratory support. Technological innovations such as non-invasive ventilation, advanced sensors, and AI-assisted controls enhance their effectiveness and adoption. The increasing number of preterm births globally is driving continuous demand for respiratory devices across NICUs.
The neonatal monitoring devices segment is expected to witness the fastest growth from 2026 to 2033, driven by the increasing need for real-time monitoring of vital signs such as heart rate, oxygen saturation, and temperature. Continuous monitoring helps clinicians detect early complications, optimize interventions, and improve neonatal outcomes. The growth is also fueled by AI integration, wireless connectivity, and telehealth capabilities that allow remote patient monitoring and centralized NICU management. Hospitals and specialized care centers are rapidly adopting these devices to enhance patient safety and reduce clinical workload. The expansion of NICU facilities in emerging markets further accelerates the demand for modern monitoring solutions.
- By Application
On the basis of application, the neonatal intensive care market is segmented into medication administration, transfusion of blood, diagnostic testing, and feeding. The medication administration segment dominated the market in 2025, as precise and timely delivery of medications is critical for neonatal survival, particularly in preterm and critically ill infants. Advanced devices and infusion systems reduce human errors, maintain accurate dosages, and ensure continuous delivery, making them indispensable in NICUs. Hospitals often prefer automated and programmable systems that integrate with electronic health records to improve workflow efficiency. The growing prevalence of neonatal complications and the emphasis on evidence-based care drive adoption in both developed and emerging regions.
The feeding segment is expected to witness the fastest growth from 2026 to 2033, fueled by the increasing focus on neonatal nutrition and growth in preterm infants. Feeding devices, including enteral feeding pumps and tube systems, enable controlled and safe nutrition delivery, supporting healthy weight gain and overall development. The segment benefits from innovations in precision feeding technologies, minimizing risks of aspiration and feeding intolerance. Rising awareness among parents and clinicians about the importance of optimized neonatal nutrition is encouraging wider adoption across hospitals and specialized neonatal care centers. Emerging economies investing in NICU infrastructure also contribute to the rapid growth of the feeding segment.
- By End User
On the basis of end user, the neonatal intensive care market is segmented into hospitals, clinics, and ambulatory surgical centers. The hospitals segment dominated the market in 2025 due to their advanced infrastructure, higher patient volumes, and well-equipped NICUs capable of handling high-risk neonates. Hospitals are the primary adopters of sophisticated NICU equipment, including incubators, respiratory devices, and monitoring systems. The presence of specialized neonatal staff, critical care protocols, and technological capabilities makes hospitals the largest revenue contributors to the market. Continuous investments in maternal and neonatal healthcare and expansion of NICU capacity in hospitals further reinforce their dominance.
The clinics segment is expected to witness the fastest growth from 2026 to 2033, driven by the increasing establishment of specialized neonatal care centers and outpatient services. Clinics provide focused neonatal support, including diagnostic testing, medication administration, and feeding assistance, catering to moderate-risk newborns and follow-up care. The growth is fueled by rising awareness among parents, expansion of private healthcare facilities, and government initiatives supporting neonatal health in urban and semi-urban regions. Clinics increasingly adopt portable and cost-effective NICU devices, making them a key growth segment in emerging markets.
Neonatal Intensive Care Market Regional Analysis
- North America dominated the neonatal intensive care market with the largest revenue share of 38.9% in 2025, characterized by advanced healthcare infrastructure, high healthcare spending, and a strong presence of key NICU equipment manufacturers, with the U.S. witnessing significant growth in NICU bed installations and adoption of cutting-edge monitoring and respiratory devices
- Hospitals in the region prioritize investment in modern incubators, respiratory devices, and neonatal monitoring systems, ensuring improved survival rates and high-quality care for preterm and critically ill newborns
- This widespread adoption is further supported by well-trained neonatal healthcare professionals, government initiatives promoting maternal and child health, and the presence of leading NICU equipment manufacturers, establishing North America as a key market for neonatal intensive care solutions
U.S. Neonatal Intensive Care Market Insight
The U.S. neonatal intensive care market captured the largest revenue share of 82% in 2025 within North America, fueled by advanced healthcare infrastructure and high-quality NICU facilities. Hospitals and specialized neonatal care centers are increasingly adopting cutting-edge incubators, respiratory devices, and monitoring systems to improve preterm and critically ill newborn survival rates. The growing awareness of early intervention and advanced neonatal care practices further drives market expansion. Moreover, government initiatives and funding programs supporting maternal and child health are promoting the establishment and upgrade of NICUs. The integration of AI-enabled monitoring and telehealth solutions in U.S. NICUs is enhancing patient outcomes and operational efficiency.
Europe Neonatal Intensive Care Market Insight
The Europe neonatal intensive care market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by stringent healthcare regulations and rising awareness about neonatal health. The increase in hospital infrastructure and specialized neonatal care units is fostering the adoption of advanced NICU equipment. European healthcare providers are increasingly focused on improving neonatal survival rates, driving demand for incubators, respiratory devices, and monitoring systems. The region is witnessing significant growth across public and private hospitals, as well as maternal and child care centers. The adoption of telehealth-enabled NICU monitoring and advanced neonatal technologies is gaining traction.
U.K. Neonatal Intensive Care Market Insight
The U.K. neonatal intensive care market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the increasing emphasis on reducing neonatal mortality and improving preterm care. Hospitals and specialized neonatal units are investing in state-of-the-art incubators, respiratory devices, and AI-enabled monitoring solutions. The rising prevalence of preterm births and neonatal complications is pushing healthcare providers to adopt advanced NICU equipment. Government health initiatives and funding schemes supporting maternal and child health further promote NICU expansion. The country’s focus on research and innovation in neonatal care is encouraging hospitals to implement technologically advanced solutions for critical care.
Germany Neonatal Intensive Care Market Insight
The Germany neonatal intensive care market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of neonatal health and demand for technologically advanced NICU solutions. Hospitals and neonatal units are adopting high-precision incubators, ventilators, and monitoring devices to enhance preterm infant survival and care quality. Germany’s strong healthcare infrastructure, emphasis on innovation, and strict regulatory compliance promote adoption of advanced NICU technologies. Integration of AI and telehealth in neonatal care is becoming increasingly prevalent. Hospitals prioritize safe, efficient, and eco-conscious solutions, aligning with consumer expectations and national healthcare goals.
Asia-Pacific Neonatal Intensive Care Market Insight
The Asia-Pacific neonatal intensive care market is poised to grow at the fastest CAGR of 23% during the forecast period of 2026 to 2033, driven by rising preterm birth rates, improving healthcare infrastructure, and government initiatives supporting maternal and child health. Countries such as China, India, and Japan are witnessing rapid expansion of NICU facilities and adoption of advanced incubators, respiratory devices, and monitoring systems. Growing awareness among parents and healthcare providers about neonatal care and early intervention is boosting market demand. The emergence of local manufacturers and cost-effective NICU solutions is increasing accessibility. Expansion of private hospitals and specialized neonatal care centers further supports market growth.
Japan Neonatal Intensive Care Market Insight
The Japan neonatal intensive care market is gaining momentum due to the country’s advanced healthcare system, high preterm birth survival focus, and technological adoption in hospitals. NICUs are increasingly equipped with AI-enabled monitoring, respiratory devices, and modern incubators to provide superior care for critically ill neonates. The integration of telehealth and IoT-based solutions in neonatal care is enhancing operational efficiency and patient outcomes. Growing demand for specialized neonatal services in both public and private hospitals supports market expansion. Moreover, Japan’s aging population and rising awareness of neonatal health are expected to further drive NICU equipment adoption.
India Neonatal Intensive Care Market Insight
The India neonatal intensive care market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rising preterm births, expanding hospital infrastructure, and increasing awareness about neonatal health. Hospitals and specialized NICU centers are investing in advanced incubators, respiratory devices, and monitoring systems to improve survival rates. Government programs promoting maternal and child health and the development of smart hospitals are boosting market growth. Affordable NICU solutions from domestic manufacturers and the increasing availability of technologically advanced equipment are driving adoption. Rapid urbanization and increasing healthcare spending are further supporting market expansion in India.
Neonatal Intensive Care Market Share
The Neonatal Intensive Care industry is primarily led by well-established companies, including:
- GE HealthCare (U.S.)
- Drägerwerk AG & Co. KGaA (Germany)
- Fisher & Paykel Healthcare Limited (New Zealand)
- Natus Medical Incorporated (U.S.)
- Medtronic plc (Ireland)
- Koninklijke Philips N.V. (Netherlands)
- Masimo Corporation (U.S.)
- BD (U.S.)
- Vyaire Medical, Inc. (U.S.)
- Smiths Medical (U.S.)
- Mindray Medical International Limited (China)
- Phoenix Medical Systems Pvt. Ltd. (India)
- Inspiration Healthcare Group plc (U.K.)
- Atom Medical Corporation (Japan)
- Fanem Ltda. (Brazil)
- Ardo medical AG (Switzerland)
- COBAMS srl (Italy)
- Nice Neotech Medical Systems Pvt. Ltd. (India)
- AVI Healthcare Pvt Ltd. (India)
- Novos Medical Systems (India)
What are the Recent Developments in Global Neonatal Intensive Care Market?
- In September 2025, a lightweight, portable Mom Incubator successfully passed clinical trials at five NHS trusts in the UK, showing high rates of thermoregulation and enabling premature infants to stay closer to their mothers rather than in traditional NICUs. Its strong performance in NHS testing highlights growing adoption of more portable neonatal care solution
- In July 2025, Medicover Hospitals in Navi Mumbai launched a state‑of‑the‑art Level III Neonatal Intensive Care Unit (NICU) as part of its new Women & Child Wing, providing advanced continuous critical care, monitoring, ventilation, and life‑support systems tailored for premature and critically ill newborns. This facility integrates antenatal through postnatal care under one roof and emphasizes family‑centered involvement during the neonatal period
- In June 2025, Baylor Scott & White Medical Center – Round Rock opened its first Level III NICU, significantly boosting local neonatal care capacity by enabling treatment of very premature infants (as early as 24 weeks gestation) without transfer to distant tertiary centers. The NICU features family‑supportive private rooms and remote camera systems that enhance care accessibility and parental engagement
- In June 2023, Dräger launched the FDA‑cleared Babyroo TN300 open care warmer designed for use from delivery rooms to NICUs, offering advanced thermoregulation and integrated respiratory support to stabilize premature infants immediately after birth. This product enhances early stabilization, supports lung protection, and aligns with family‑centered neonatal care practices
- In April 2022, the World Health Organization (WHO) released the second edition of its Essential Newborn Care Course (ENCC), updating the global training curriculum with competency‑based modules and structured simulation to improve newborn care standards and quality across health systems. This update aims to strengthen neonatal care practices worldwide through standardized education and data‑driven quality improvement
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.