Global Neurofibromatosis Type 1 Market
Market Size in USD Million
CAGR :
% 
 USD
343.30 Million
USD
735.89 Million
2024
2032
USD
343.30 Million
USD
735.89 Million
2024
2032
| 2025 –2032 | |
| USD 343.30 Million | |
| USD 735.89 Million | |
|
|
|
|
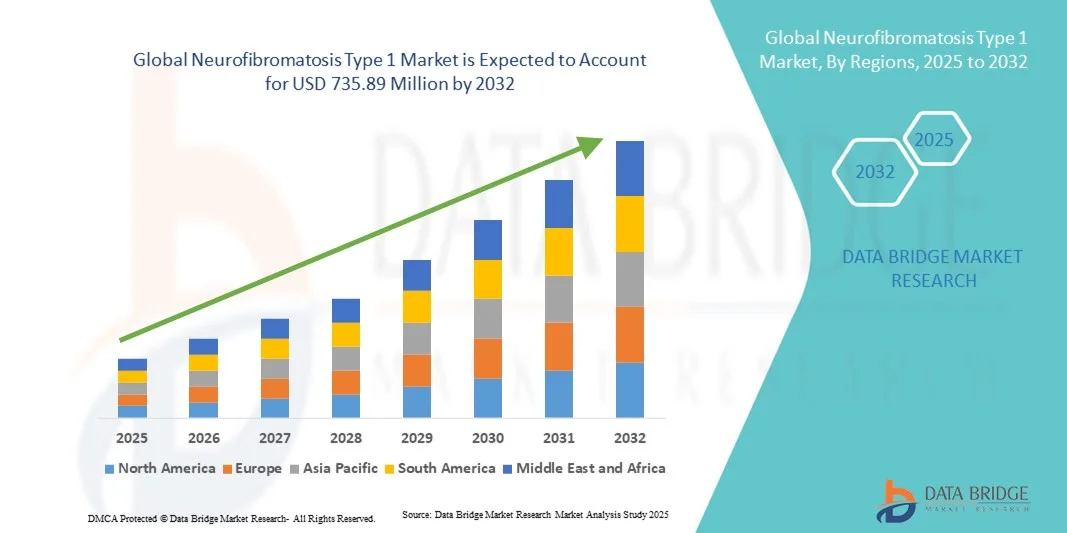
Neurofibromatosis Type 1 Market Size
- The global neurofibromatosis type 1 market size was valued at USD 343.30 million in 2024 and is expected to reach USD 735.89 million by 2032, at a CAGR of 10.00% during the forecast period
- The market growth is primarily driven by increasing awareness of genetic disorders, advancements in diagnostic techniques, and the rising development of targeted therapies addressing neurofibromin-related mutations
- Furthermore, growing investments in rare disease research, expanding clinical trials for novel treatment options, and enhanced patient support programs are accelerating therapeutic innovation, thereby significantly propelling the global neurofibromatosis type 1 market growth.
Neurofibromatosis Type 1 Market Analysis
- Neurofibromatosis type 1 (NF1), a rare genetic disorder caused by mutations in the NF1 gene leading to benign and malignant tumor formation, is increasingly recognized as a critical focus area in rare disease research due to its complex clinical manifestations and rising demand for effective management options
- The growing market demand is primarily driven by advancements in molecular diagnostics, increasing awareness among healthcare professionals, and the development of targeted drug therapies aimed at slowing tumor growth and improving quality of life
- North America dominated the neurofibromatosis type 1 market with the largest revenue share of 41.2% in 2024, supported by strong clinical research infrastructure, growing orphan drug approvals, and a high rate of patient identification through genetic testing
- Asia-Pacific is expected to be the fastest-growing region during the forecast period due to improving healthcare access, expanding genetic testing availability, and increasing government focus on rare disease management programs
- Drug therapy segment dominated the neurofibromatosis type 1 market with a market share of 47.3% in 2024, driven by the growing adoption of targeted treatments such as MEK inhibitors and ongoing advancements in pharmacological research aimed at managing tumor progression and related complications
Report Scope and Neurofibromatosis Type 1 Market Segmentation
|
Attributes |
Neurofibromatosis Type 1 Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Neurofibromatosis Type 1 Market Trends
Advancements in Targeted and Genetic Therapies
- A significant and accelerating trend in the global neurofibromatosis type 1 (NF1) market is the rapid development of targeted therapies and gene-based treatments addressing the underlying NF1 gene mutation. This evolution in therapeutic approaches is transforming disease management and patient outcomes
- For instance, the approval of selumetinib (Koselugo) for pediatric NF1 patients with inoperable plexiform neurofibromas marked a major breakthrough, highlighting the growing clinical success of MEK inhibitors. Similarly, ongoing research into CRISPR-based gene editing and RNA therapies aims to correct or suppress the faulty NF1 gene function
- AI-assisted diagnostic tools are being increasingly used to improve the early identification of NF1 manifestations and to monitor tumor progression more effectively. For instance, advanced imaging platforms integrated with AI can detect subtle lesion changes and predict tumor behavior, enhancing personalized treatment planning. Furthermore, molecular profiling of NF1 patients enables precision-targeted therapy development, optimizing treatment efficacy
- The integration of genomics, molecular biology, and AI-driven analytics is streamlining the discovery of novel biomarkers and therapeutic targets for NF1. Through these combined technologies, researchers are gaining deeper insight into disease mechanisms, thereby facilitating faster drug development and better patient stratification in clinical trials
- This trend toward personalized, gene-focused, and data-driven approaches is reshaping the global NF1 treatment landscape. Consequently, companies such as AstraZeneca, SpringWorks Therapeutics, and NFlection Therapeutics are expanding their R&D pipelines for MEK inhibitors and novel gene therapies targeting neurofibromin restoration
- The growing adoption of advanced diagnostics and gene-based therapeutics is expected to drive robust market expansion as healthcare providers increasingly prioritize precision medicine and early intervention for NF1 management
Neurofibromatosis Type 1 Market Dynamics
Driver
Growing Demand for Targeted Treatments and Early Diagnosis
- The increasing recognition of neurofibromatosis type 1 as a significant rare genetic disorder, coupled with advancements in molecular diagnostics, is a key driver for market growth
- For instance, in March 2024, AstraZeneca announced continued clinical success of selumetinib for NF1-related tumors, reinforcing the demand for targeted therapies and highlighting industry commitment to innovation
- As awareness of NF1’s neurological and oncological complications grows, patients and clinicians are increasingly seeking precise diagnostic and therapeutic solutions, strengthening healthcare investment in early detection
- Furthermore, the expansion of national rare disease programs and growing collaboration between biotech companies and research institutes are enhancing drug development efforts, accelerating treatment availability across major regions
- The growing patient focus on improved quality of life and long-term disease control through minimally invasive therapies is further fueling market expansion, supported by favorable reimbursement frameworks in developed economies
- For instance, partnerships between pharmaceutical firms and academic centers such as the Children’s Tumor Foundation are expediting translational research and accelerating new drug discovery for NF1
- Technological advances in next-generation sequencing (NGS) and AI-based diagnostic platforms are also driving early and accurate identification of NF1, supporting timely therapeutic intervention and patient management
Restraint/Challenge
High Treatment Costs and Limited Awareness in Developing Regions
- The elevated cost associated with targeted and genetic therapies for NF1 presents a significant challenge to widespread treatment accessibility and market penetration
- For instance, MEK inhibitors such as selumetinib remain expensive, with limited insurance coverage in several low- and middle-income countries, creating disparities in patient care
- Insufficient awareness and diagnostic infrastructure in developing regions lead to underdiagnosis and delayed treatment initiation, hindering early disease management and clinical outcomes
- Addressing these barriers through government-led rare disease initiatives, expanded insurance coverage, and global nonprofit partnerships is critical for ensuring broader access to NF1 treatments. In addition, complex regulatory pathways for orphan drugs can delay product approvals, impacting timely market entry for innovative therapies
- While public-private partnerships and patient advocacy groups are increasingly driving awareness, sustained investment in affordable diagnostics and treatment accessibility will be essential for overcoming existing challenges and achieving equitable global NF1 care
- For instance, delays in reimbursement approvals for orphan drugs in emerging markets limit the uptake of advanced NF1 treatments despite growing patient need
- The scarcity of specialized clinicians trained in managing NF1 and its associated complications further restricts patient access to optimal care, particularly in resource-limited healthcare settings
Neurofibromatosis Type 1 Market Scope
The market is segmented on the basis of treatment, end user, and distribution channel.
- By Treatment
On the basis of treatment, the neurofibromatosis type 1 market is segmented into drug therapy, surgery, radiation therapy, and chemotherapy. The drug therapy segment dominated the market with the largest revenue share of 47.3% in 2024, driven by the increasing adoption of targeted therapies such as MEK inhibitors (e.g., selumetinib) that directly address the molecular mechanisms of NF1. The growing clinical evidence supporting the efficacy of pharmacological treatments in reducing the size of plexiform neurofibromas and improving quality of life has accelerated their use among both pediatric and adult patients. Supportive regulatory frameworks for orphan drugs and continuous R&D investments by pharmaceutical companies have also contributed to the dominance of this segment. Furthermore, the non-invasive nature of drug therapy, along with growing patient preference for oral and manageable treatment options, reinforces its leadership in the global market.
The surgery segment is expected to witness the fastest growth rate from 2025 to 2032, attributed to the increasing incidence of complex neurofibromas requiring surgical removal and improvements in microsurgical and neuro-oncological techniques. Advanced imaging guidance and minimally invasive procedures are enhancing surgical precision and reducing recovery time, making this an attractive treatment option for severe NF1 cases. For instance, multidisciplinary surgical approaches combining plastic surgery and neurosurgery have shown success in reducing complications and improving cosmetic outcomes. The rising availability of specialized surgeons and improved hospital infrastructure in emerging economies further supports the rapid growth of the surgical segment.
- By End User
On the basis of end user, the neurofibromatosis type 1 market is segmented into hospitals, specialty clinics, ambulatory surgical centers, and others. The hospitals segment dominated the market with the largest revenue share in 2024, owing to their comprehensive diagnostic and treatment capabilities, availability of multidisciplinary teams, and advanced imaging and genetic testing facilities. Hospitals are the primary point of care for NF1 patients, especially for those undergoing surgeries or clinical trial participation for emerging therapies. Increasing collaborations between hospitals and research organizations for rare disease studies and drug testing further bolster this segment’s dominance. Moreover, hospitals often serve as centers for genetic counseling and long-term monitoring of NF1 complications, reinforcing their central role in patient management.
The specialty clinics segment is projected to register the fastest growth rate from 2025 to 2032, driven by the growing establishment of dedicated neurofibromatosis and genetic disorder clinics focusing on individualized care. These centers offer focused expertise in genetic counseling, advanced diagnostics, and therapeutic planning, attracting patients seeking specialized attention. The expansion of private specialty clinics with access to clinical trials and novel drug therapies is further accelerating this segment’s growth. In addition, the increasing availability of telehealth services through specialty centers enables continuous care for NF1 patients in remote or underserved regions.
- By Distribution Channel
On the basis of distribution channel, the neurofibromatosis type 1 market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment held the largest market share in 2024, supported by the direct availability of specialized NF1 medications and the close integration of pharmacies within hospital-based treatment programs. Patients undergoing targeted or combination therapies for NF1 often rely on hospital pharmacies for timely access to prescribed drugs, dosage adjustments, and adherence support. Hospital-based distribution also ensures proper handling of orphan drugs that require strict storage and dispensing conditions. The presence of trained pharmacists familiar with rare disease treatments enhances patient safety and compliance, maintaining hospital pharmacies’ leadership in the distribution landscape.
The online pharmacy segment is anticipated to witness the fastest CAGR from 2025 to 2032, driven by the growing digitalization of healthcare and patient preference for convenient access to rare disease medications. For instance, leading e-pharmacy platforms are increasingly partnering with pharmaceutical companies to distribute orphan drugs directly to patients with verified prescriptions. The online channel supports home delivery, teleconsultation integration, and medication adherence programs, which are particularly valuable for patients requiring continuous therapy. In addition, expanding internet penetration, favorable regulatory changes supporting online drug sales, and the growing role of digital health ecosystems are expected to further accelerate this segment’s growth trajectory.
Neurofibromatosis Type 1 Market Regional Analysis
- North America dominated the neurofibromatosis type 1 market with the largest revenue share of 41.2% in 2024, supported by strong clinical research infrastructure, growing orphan drug approvals, and a high rate of patient identification through genetic testing
- Patients and healthcare providers in the region highly value precision medicine approaches, advanced diagnostic tools, and access to specialized treatment centers dedicated to rare genetic disorders such as NF1
- This strong market position is further supported by high healthcare expenditure, favorable government initiatives for orphan drug development, and extensive clinical trial activity led by major pharmaceutical and biotechnology companies, solidifying North America as the leading hub for NF1 research and treatment
U.S. Neurofibromatosis Type 1 Market Insight
The U.S. neurofibromatosis type 1 market captured the largest revenue share of 82% in 2024 within North America, fueled by strong research funding, advanced diagnostic infrastructure, and widespread access to genetic testing. Patients are increasingly benefiting from early diagnosis and innovative treatment options such as MEK inhibitors and gene-targeted therapies. The presence of major pharmaceutical companies, robust clinical trial networks, and patient advocacy organizations further strengthens the country’s market position. Moreover, supportive regulatory policies by the FDA and government-led rare disease initiatives are propelling continued therapeutic development and adoption.
Europe Neurofibromatosis Type 1 Market Insight
The Europe neurofibromatosis type 1 market is projected to expand at a steady CAGR throughout the forecast period, driven by favorable government funding for rare disease research and advancements in molecular diagnostics. The region’s strong emphasis on early genetic screening and patient registries supports improved disease management. European healthcare systems are also prioritizing equitable access to orphan drugs, contributing to broader treatment availability. Growing collaborations between research institutions and biotechnology firms are fueling innovation, while an increasing number of NF1-focused clinical centers enhance patient care quality across the continent.
U.K. Neurofibromatosis Type 1 Market Insight
The U.K. neurofibromatosis type 1 market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by the expanding National Genomic Medicine Service and the integration of precision medicine into routine care. The country’s well-established healthcare infrastructure facilitates early NF1 diagnosis through genetic testing programs. For instance, collaborations between the NHS and academic research centers are accelerating clinical trials for targeted therapies. Rising public awareness of rare genetic conditions and increased government support for orphan drug development are expected to drive significant growth in the U.K. market.
Germany Neurofibromatosis Type 1 Market Insight
The Germany neurofibromatosis type 1 market is expected to expand at a considerable CAGR during the forecast period, fueled by the nation’s strong biotechnology sector and emphasis on translational research. Germany’s advanced clinical trial environment and specialized neuro-oncology centers promote the adoption of innovative treatments such as MEK inhibitors and gene-based therapies. The country’s robust healthcare reimbursement framework and focus on personalized medicine further enhance treatment accessibility. Moreover, increasing collaborations between hospitals, universities, and pharmaceutical firms are reinforcing Germany’s leadership in NF1-related therapeutic advancements.
Asia-Pacific Neurofibromatosis Type 1 Market Insight
The Asia-Pacific neurofibromatosis type 1 market is poised to grow at the fastest CAGR of 23.8% during the forecast period of 2025 to 2032, driven by expanding healthcare infrastructure, improving genetic testing availability, and rising awareness of rare diseases. Countries such as China, Japan, and India are witnessing increased participation in global clinical trials and stronger government support for rare disease treatment access. Moreover, the presence of emerging biotechnology firms and growing collaborations with international research institutions are enhancing innovation. Expanding patient advocacy networks and digital healthcare initiatives are also facilitating earlier diagnosis and management of NF1.
Japan Neurofibromatosis Type 1 Market Insight
The Japan neurofibromatosis type 1 market is gaining momentum due to its highly developed medical research environment and commitment to precision medicine. Japanese healthcare providers emphasize early diagnosis through genetic screening and imaging technologies. For instance, research collaborations between academic hospitals and biotech firms are advancing gene therapy and MEK inhibitor trials. The country’s focus on patient safety, innovation, and integration of AI-based diagnostic platforms supports efficient NF1 management. In addition, government-backed funding for rare disease research is accelerating the introduction of novel treatment options in Japan.
India Neurofibromatosis Type 1 Market Insight
The India neurofibromatosis type 1 market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to growing awareness, a rapidly expanding healthcare sector, and increased investment in rare disease diagnostics. The nation’s expanding middle class and improved access to specialty clinics are encouraging early diagnosis and treatment. For instance, collaborations between government programs and nonprofit organizations are enhancing patient identification and data collection for NF1. The availability of affordable generic medications, growing medical tourism, and strengthening biotechnology research capacity are key factors driving the market’s robust growth in India.
Neurofibromatosis Type 1 Market Share
The Neurofibromatosis Type 1 industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- AstraZeneca (U.K.)
- Takeda Pharmaceutical Company Limited (Japan)
- Boehringer Ingelheim International GmbH (Germany)
- Vertex Pharmaceuticals Incorporated (U.S.)
- Shanghai Fosun Pharmaceutical (Group) Co., Ltd (China)
- BeiGene Ltd. (China)
- BioMarin Pharmaceutical, Inc. (U.S.)
- Exelixis, Inc. (U.S.)
- Blueprint Medicines Corporation (U.S.)
- Healx (U.K.)
- NFlection Therapeutics, Inc. (U.S.)
- PASITHEA THERAPEUTICS (U.S.)
- Infixion Bioscience (U.K.)
- Mulberry Biotherapeutics, Inc. (U.S.)
- CureAge Therapeutics (U.K.)
- Bayer AG (Germany)
What are the Recent Developments in Global Neurofibromatosis Type 1 Market?
- In July 2025, researchers at the Indiana University School of Medicine identified a novel molecular pathway responsible for the breakdown of neurofibromin, the protein whose loss underlies NF1. Blocking this pathway in NF1 mouse models led to improvements in key neurobehavioral symptoms such as hyperactivity, impulsivity, and impaired social interaction
- In February 2025, the U.S. Food and Drug Administration (FDA) granted approval for Mirdametinib (brand name Gomekli), developed by SpringWorks Therapeutics, for the treatment of adults and pediatric patients aged two years and older with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN). This marks a major milestone as Mirdametinib becomes one of the few targeted therapies available for NF1
- In February 2025, Healx, a U.K.-based AI-powered drug discovery company, announced in February 2025 that it had dosed the first patient in its Phase 2 clinical trial evaluating HLX-1502 for the treatment of neurofibromatosis type 1. The investigational therapy was identified through Healx’s artificial intelligence platform designed to repurpose and combine existing compounds for rare diseases
- In January 2025, the Children’s Tumor Foundation awarded new Drug Discovery Initiative (DDI) grants to research teams focusing on innovative therapies for NF1-related complications, including malignant peripheral nerve sheath tumors (MPNSTs). Projects involved testing the combined inhibition of BET and PARP pathways (BETi/PARPi), showing promising preclinical activity against aggressive NF1-driven cancers
- In May 2024, the Children’s Tumor Foundation (CTF) collaborated with the Global Clinical Advisory Research (GCAR) network to initiate the world’s first multi-arm, multi-manifestation platform trial for neurofibromatosis type 1 and related disorders. This innovative clinical trial framework enables simultaneous evaluation of multiple therapies under one protocol, significantly accelerating drug testing timelines
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.