Global Neurotherapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
7.88 Billion
USD
11.54 Billion
2025
2033
USD
7.88 Billion
USD
11.54 Billion
2025
2033
| 2026 –2033 | |
| USD 7.88 Billion | |
| USD 11.54 Billion | |
|
|
|
|
Neurotherapeutics Market Size
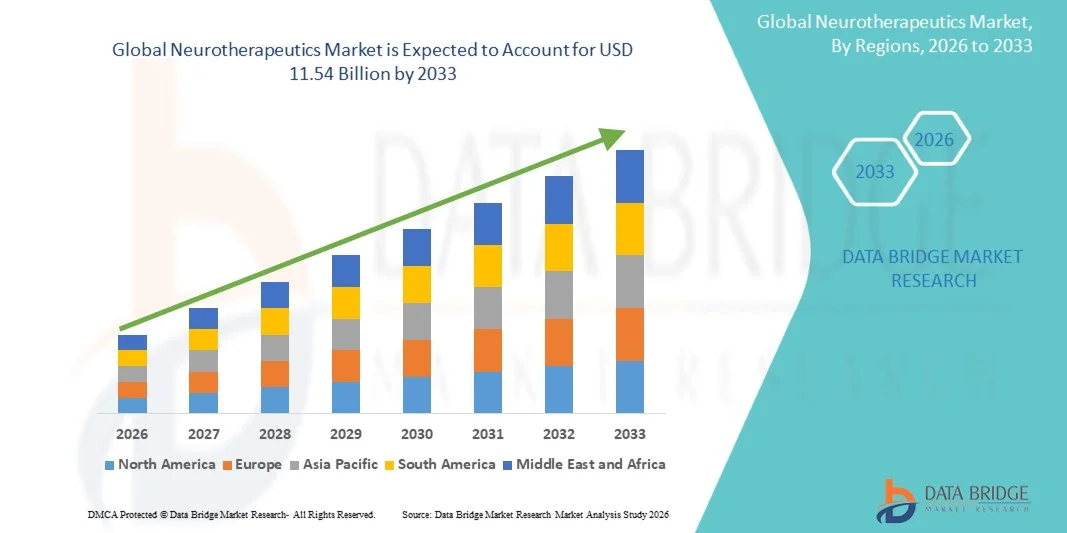
- The global neurotherapeutics market size was valued at USD 7.88 billion in 2025 and is expected to reach USD 11.54 billion by 2033, at a CAGR of 4.89% during the forecast period
- The market growth is primarily driven by increasing prevalence of neurological disorders, advancements in neuropharmacology, and the rising adoption of innovative therapies such as biologics, gene therapy, and neurostimulation devices
- Moreover, growing awareness among patients and healthcare providers about effective treatment options, coupled with supportive government initiatives and funding for neurological research, is fueling the demand for neurotherapeutic solutions across global healthcare settings. These factors are collectively propelling market expansion, making neurotherapeutics a critical segment within the pharmaceutical and medical devices industry
Neurotherapeutics Market Analysis
- Neurotherapeutics, encompassing neurological drugs and devices, are increasingly critical in managing a wide range of neurological disorders, including central nervous system (CNS), peripheral nervous system (PNS), and autonomic nervous system (ANS) disorders, due to their ability to improve patient outcomes, enhance quality of life, and provide targeted disease management
- The rising demand for neurotherapeutics is primarily driven by the increasing prevalence of neurological disorders, growing awareness among patients and healthcare providers about advanced treatment options, and technological innovations in drug development, neurostimulation devices, and precision therapy solutions
- North America dominated the neurotherapeutics market with the largest revenue share of 38.7% in 2025, supported by strong healthcare infrastructure, high R&D investment, early adoption of innovative therapies, and a robust presence of key pharmaceutical and medical device companies, with the U.S. witnessing significant growth in clinical trials and neurotherapeutic prescriptions
- Asia-Pacific is expected to be the fastest growing region in the neurotherapeutics market during the forecast period due to rising prevalence of neurological disorders, increasing healthcare spending, improving medical infrastructure, and expanding access to neurological drugs and devices in emerging economies such as China and India
- Neurological drugs dominated the neurotherapeutics market with a market share of 45.3% in 2025, driven by their established treatment protocols, wide availability across hospitals and neurotherapeutic centers, and continuous development of novel drugs targeting complex neurological conditions
Report Scope and Neurotherapeutics Market Segmentation
|
Attributes |
Neurotherapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Neurotherapeutics Market Trends
“Personalized Neurotherapeutics and AI-Enabled Monitoring”
- A significant and accelerating trend in the global neurotherapeutics market is the growing adoption of personalized therapies and AI-enabled monitoring systems, which allow treatments to be tailored to individual patient profiles for better efficacy and safety
- For instance, AI-powered platforms such as Neuromatch and BrainQ can analyze patient data to optimize neurostimulation parameters and predict therapeutic outcomes, improving treatment precision
- Integration of AI in neurotherapeutics enables features such as real-time monitoring of patient responses, adaptive dosing recommendations, and predictive alerts for potential adverse events, enhancing clinical decision-making
- Personalized neurotherapeutics combined with digital health platforms facilitates centralized management of treatment plans, allowing healthcare providers to coordinate drug therapies, device interventions, and rehabilitation protocols in a unified approach
- Growing partnerships between pharmaceutical companies and digital health startups are accelerating the development of smart neurotherapeutic solutions with integrated data analytics
- Telemedicine-enabled neurotherapeutics is emerging as a trend, allowing remote patient monitoring and virtual adjustments to treatment plans, particularly for chronic neurological conditions
- This trend towards more individualized, data-driven, and technology-assisted neurotherapeutic solutions is reshaping patient and clinician expectations for neurological care
- The demand for AI-enabled and personalized neurotherapeutic solutions is growing rapidly across hospitals, neurotherapeutic centers, and research institutions, as patients increasingly seek optimized outcomes and minimized side effects
Neurotherapeutics Market Dynamics
Driver
“Increasing Prevalence of Neurological Disorders and Technological Advancements”
- The rising incidence of CNS, PNS, and ANS disorders, coupled with continuous innovations in neurological drugs and devices, is a key driver for the growing adoption of neurotherapeutics
- For instance, in March 2025, Biogen announced an advancement in AI-assisted neurostimulation therapy for Alzheimer’s patients, highlighting the role of technology in improving clinical outcomes
- As awareness of neurological conditions increases, patients and healthcare providers are seeking more effective therapies that offer targeted interventions and improved quality of life
- Moreover, advancements in neuropharmacology, gene therapies, and non-invasive neurostimulation devices are making neurotherapeutics increasingly accessible and effective for complex neurological disorders
- The growing focus on integrated healthcare solutions, including combination therapies and remote monitoring, is further propelling the adoption of neurotherapeutic treatments across hospitals, specialized centers, and clinical research organizations
- Expansion of government-funded neurological research programs and initiatives to support patient access to innovative therapies is boosting market growth
- Increasing collaborations between hospitals, pharmaceutical companies, and research organizations are accelerating clinical trials and faster commercialization of novel neurotherapeutic solutions
Restraint/Challenge
“High Treatment Costs and Regulatory Hurdles”
- The high costs associated with advanced neurotherapeutic drugs and devices, coupled with stringent regulatory requirements, pose significant challenges to widespread market adoption
- For instance, novel gene therapies and implantable neurostimulation devices often involve multi-stage clinical trials and complex approval processes, limiting their rapid availability
- Ensuring patient safety through rigorous testing, compliance with regulatory standards, and post-market surveillance is critical but can delay product launches and increase development costs
- The need for specialized healthcare infrastructure and trained professionals to administer complex neurotherapeutics further constrains accessibility, particularly in emerging economies
- While some generic or off-patent neurological drugs help reduce costs, premium neurotherapeutic solutions with advanced features remain financially inaccessible to many patients, affecting adoption rates
- Lack of harmonized regulatory frameworks across countries can delay international market expansion for new neurotherapeutics
- Patient adherence challenges due to complex therapy regimens, frequent hospital visits, or invasive device procedures can limit the overall effectiveness and uptake of neurotherapeutic solutions
- Overcoming these challenges through cost-effective therapy development, streamlined regulatory pathways, and expanded healthcare infrastructure is vital for sustainable growth in the neurotherapeutics market
Neurotherapeutics Market Scope
The market is segmented on the basis of treatment, disorders, and distribution channel.
- By Treatment
On the basis of treatment, the neurotherapeutics market is segmented into neurological drugs and neurological devices. The neurological drugs segment dominated the market with the largest revenue share of 45.3% in 2025, driven by the widespread use of pharmacological therapies for CNS, PNS, and ANS disorders. Neurological drugs are considered the first-line treatment for conditions such as epilepsy, Parkinson’s, Alzheimer’s, and neuropathic pain, with extensive clinical evidence supporting their efficacy. Hospitals and neurotherapeutic centers often prioritize drug therapies due to their ease of administration, standardized dosing, and well-established treatment protocols. The availability of both branded and generic drugs further supports adoption and accessibility. The segment also benefits from continuous research and development, leading to next-generation drugs with improved efficacy and safety profiles. In addition, neurological drugs are compatible with combination therapy approaches, allowing personalized treatment plans alongside device-based interventions.
The neurological devices segment is anticipated to witness the fastest growth rate from 2026 to 2033, driven by rising adoption of neurostimulation, wearable monitoring, and implantable therapeutic devices. Devices such as deep brain stimulators, vagus nerve stimulators, and non-invasive neurostimulation platforms are increasingly used for treatment-resistant neurological disorders. Technological advancements in miniaturization, wireless connectivity, and AI-enabled personalization are boosting the appeal of devices. Hospitals and specialized neurotherapeutic centers are adopting these solutions for precision therapy and real-time monitoring. The growth is further supported by increasing awareness among patients seeking alternatives to long-term medication. Favorable reimbursement policies in developed regions and growing clinical trial pipelines for device-based therapies also contribute to the rapid expansion of this segment.
- By Disorders
On the basis of disorders, the neurotherapeutics market is segmented into CNS disorders, PNS disorders, and ANS disorders. The CNS disorders segment dominated the market in 2025 due to the high prevalence of neurological conditions such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and epilepsy. CNS disorders often require long-term, multidisciplinary treatment approaches combining drugs, devices, and rehabilitation programs, which drives sustained demand. Hospitals, neurotherapeutic centers, and clinical research organizations are key end-users for CNS disorder therapies, given the complexity of management. The segment is supported by robust R&D activity in both pharmaceutical and device-based treatments. Aging populations in developed countries are a major contributor to the growing CNS disorder burden. Furthermore, CNS disorder treatments are often complemented by advanced diagnostic and monitoring technologies, increasing the integration of neurotherapeutics in clinical practice.
The PNS disorders segment is expected to witness the fastest growth rate from 2026 to 2033, fueled by the rising prevalence of peripheral neuropathies, diabetic neuropathy, and nerve injury cases worldwide. Increasing awareness of peripheral nerve disorders and demand for targeted neurotherapeutic interventions are boosting adoption. Neurological drugs and nerve stimulation devices designed for PNS disorders offer improved patient outcomes with fewer systemic side effects. Emerging markets with expanding healthcare infrastructure are contributing to faster growth. Moreover, advances in minimally invasive neuromodulation devices and wearable therapies are enhancing treatment accessibility and compliance. The segment also benefits from ongoing clinical trials and regulatory approvals for innovative PNS-targeted therapies.
- By Distribution Channel
On the basis of distribution channel, the neurotherapeutics market is segmented into hospitals, neurotherapeutic centers, clinical research organizations, and others. The hospitals segment dominated the market in 2025, attributed to the availability of specialized neurologists, advanced diagnostic tools, and integrated treatment services. Hospitals are the primary channel for administering both drug and device-based therapies for CNS, PNS, and ANS disorders. Patients often prefer hospital-based treatment due to the presence of multidisciplinary teams capable of handling complex neurological conditions. Hospitals also facilitate participation in clinical trials, providing access to emerging therapies. The large patient base and established reimbursement frameworks further support revenue generation through hospital channels. In addition, hospitals often collaborate with pharmaceutical companies and device manufacturers for early access programs and training initiatives.
The neurotherapeutic centers segment is expected to witness the fastest growth rate from 2026 to 2033, driven by increasing specialization in neurological care and outpatient management services. These centers focus on personalized care, advanced neurostimulation, and rehabilitation therapies, offering convenient and continuous monitoring for patients. Expansion of neurotherapeutic centers in emerging markets is improving access to high-quality neurological treatments. Adoption of digital health platforms and AI-enabled monitoring systems in these centers is enhancing treatment precision. The segment benefits from increased patient awareness and preference for focused care outside traditional hospital settings. Strategic partnerships with pharmaceutical and device companies for clinical research and therapy delivery further accelerate growth in this channel.
Neurotherapeutics Market Regional Analysis
- North America dominated the neurotherapeutics market with the largest revenue share of 38.7% in 2025, supported by strong healthcare infrastructure, high R&D investment, early adoption of innovative therapies, and a robust presence of key pharmaceutical and medical device companies, with the U.S. witnessing significant growth in clinical trials and neurotherapeutic prescriptions
- Patients and healthcare providers in the region increasingly prefer advanced neurotherapeutic solutions, including neurological drugs and neurostimulation devices, due to their proven efficacy, targeted action, and ability to improve patient outcomes for CNS, PNS, and ANS disorders
- This widespread adoption is further supported by well-established reimbursement policies, a technologically adept medical workforce, and the presence of leading global neurotherapeutics companies, establishing North America as a critical hub for both treatment access and ongoing clinical research in neurological care
U.S. Neurotherapeutics Market Insight
The U.S. neurotherapeutics market captured the largest revenue share of 42% in 2025 within North America, fueled by the high prevalence of neurological disorders such as Alzheimer’s, Parkinson’s, and epilepsy. Patients and healthcare providers increasingly prioritize advanced neurological drugs and neurostimulation devices due to their targeted efficacy and potential to improve patient outcomes. The growing adoption of AI-assisted monitoring, personalized therapies, and telemedicine-enabled neurotherapeutic solutions further propels market growth. Moreover, robust R&D investment, well-established clinical trial infrastructure, and favorable reimbursement policies are significantly contributing to the market’s expansion.
Europe Neurotherapeutics Market Insight
The Europe neurotherapeutics market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing awareness of neurological disorders, supportive healthcare policies, and the demand for advanced treatment options in hospitals and specialized neurotherapeutic centers. Rising urbanization and an aging population are fueling the prevalence of CNS, PNS, and ANS disorders, encouraging the adoption of neurotherapeutic solutions. The region is experiencing significant growth across hospital and outpatient care settings, with both drug and device-based therapies being integrated into treatment plans for chronic neurological conditions.
U.K. Neurotherapeutics Market Insight
The U.K. neurotherapeutics market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the rising incidence of neurological disorders and increased focus on early diagnosis and personalized treatment. Concerns regarding cognitive decline, nerve disorders, and mobility-related conditions are encouraging patients and healthcare providers to adopt advanced drug therapies and neurostimulation devices. The U.K.’s robust healthcare infrastructure, combined with strong clinical research activity and increasing availability of digital health platforms, continues to stimulate market growth.
Germany Neurotherapeutics Market Insight
The Germany neurotherapeutics market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of neurological health, growing adoption of technologically advanced neurostimulation devices, and the demand for innovative pharmacological therapies. Germany’s well-developed healthcare infrastructure, focus on clinical innovation, and emphasis on patient-centric care promote the use of both hospital-based and outpatient neurotherapeutic solutions. Integration of digital health platforms for monitoring and personalized treatment plans is becoming increasingly prevalent, aligning with local patient expectations for quality care.
Asia-Pacific Neurotherapeutics Market Insight
The Asia-Pacific neurotherapeutics market is poised to grow at the fastest CAGR of 22% during the forecast period of 2026 to 2033, driven by increasing prevalence of neurological disorders, rapid urbanization, rising disposable incomes, and expanding healthcare infrastructure in countries such as China, Japan, and India. Government initiatives promoting healthcare access and digital health adoption are supporting market growth. Furthermore, the increasing number of specialized neurotherapeutic centers and expanding clinical research programs across the region are improving accessibility to advanced neurological drugs and devices.
Japan Neurotherapeutics Market Insight
The Japan neurotherapeutics market is gaining momentum due to the country’s high prevalence of age-related neurological disorders, technologically advanced healthcare infrastructure, and focus on patient-centered care. Growth is driven by increasing adoption of neurostimulation devices, AI-assisted therapy platforms, and integration of digital monitoring solutions into patient management. Moreover, Japan’s aging population is expected to drive demand for therapies that improve mobility, cognitive function, and overall quality of life in both residential and clinical settings.
India Neurotherapeutics Market Insight
The India neurotherapeutics market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to the country’s expanding healthcare access, growing prevalence of neurological disorders, and rising patient awareness of advanced therapies. India is emerging as a key market for both neurological drugs and devices, supported by government initiatives for neurological health, increasing investment in clinical research organizations, and strong domestic pharmaceutical manufacturing. The affordability of therapies, coupled with expanding hospital and neurotherapeutic center networks, is propelling market adoption across residential, outpatient, and clinical settings.
Neurotherapeutics Market Share
The Neurotherapeutics industry is primarily led by well-established companies, including:
- Medtronic (Ireland)
- Neurocrine Biosciences, Inc. (U.S.)
- Biogen Inc. (U.S.)
- Novartis AG (Switzerland)
- Teva Pharmaceutical Industries Ltd (Israel)
- Eli Lilly and Company (U.S.)
- Sage Therapeutics (U.S.)
- Aleva Neurotherapeutics (Switzerland)
- LivaNova PLC (U.K.)
- Stryker (U.S.)
- NeuroSigma, Inc. (U.S.)
- Alector (U.S.)
- Cerevel Therapeutics (U.S.)
- Axsome Therapeutics, Inc. (U.S.)
- UCB (Belgium)
- Lundbeck A/S (Denmark)
- NeuroPace (U.S.)
- Neuronetics (U.S.)
- Boston Scientific Corporation (U.S.)
- Abbott (U.S.)
What are the Recent Developments in Global Neurotherapeutics Market?
- In December 2025, the U.S. Food and Drug Administration (FDA) approved Flow Neuroscience’s FL‑100, the first brain stimulation device for at‑home treatment of depression, enabling adults with moderate to severe major depressive disorder to receive non‑invasive electrical brain stimulation under remote supervision marking a breakthrough in patient‑centric neurotherapeutic devices
- In September 2025, Relief Therapeutics and NeuroX announced a business combination to form a new digital neurotherapeutics company, aiming to expand digital therapeutic offerings for neurological care and accelerate innovation a key strategic shift toward integrated digital solutions in neurotherapeutic treatment paradigms
- In April 2025, the FDA approved a prefilled syringe self‑injection version of VYVGART Hytrulo (efgartigimod alfa + hyaluronidase‑qvfc) for adult patients with generalized myasthenia gravis (gMG) and chronic inflammatory demyelinating polyneuropathy (CIDP), allowing patients to administer treatment outside clinical settings a significant shift toward more accessible neurotherapeutic care
- In February 2025, the FDA granted approval for an adaptive brain pacemaker developed by Medtronic to treat Parkinson’s disease, a device that dynamically adjusts stimulation based on real‑time needs, improving symptom control for patients and representing a major advancement in neurostimulative technologies
- In January 2025, Johnson & Johnson (J&J) announced a plan to acquire Intra‑Cellular Therapies in a USD 14.6 billion deal, significantly expanding its neurological and psychiatric drug portfolio particularly strengthening its position in treatments such as Caplyta for schizophrenia and bipolar depression, emphasizing strategic consolidation in neurotherapeutic pharmaceuticals
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.