Global Next Gen Sequencing For Rare Disease Diagnosis Market
Market Size in USD Million
CAGR :
% 
 USD
1.44 Million
USD
2.68 Million
2024
2032
USD
1.44 Million
USD
2.68 Million
2024
2032
| 2025 –2032 | |
| USD 1.44 Million | |
| USD 2.68 Million | |
|
|
|
|
Next-Gen Sequencing for Rare Disease Diagnosis Market Analysis
The global next-gen sequencing (NGS) for rare disease diagnosis market is expanding rapidly due to the increasing use of NGS in diagnosing rare genetic disorders such as cystic fibrosis and Duchenne muscular dystrophy, which affect about 1 in 12 individuals globally. NGS technologies, such as whole genome and exome sequencing, enable accurate detection of mutations, driving better clinical outcomes and personalized treatments. With rising awareness and research into rare diseases, NGS is becoming essential for diagnosing complex neurological and genetic conditions, especially in regions such as North America, Europe, and Asia-Pacific.
Next-Gen Sequencing for Rare Disease Diagnosis Market Size
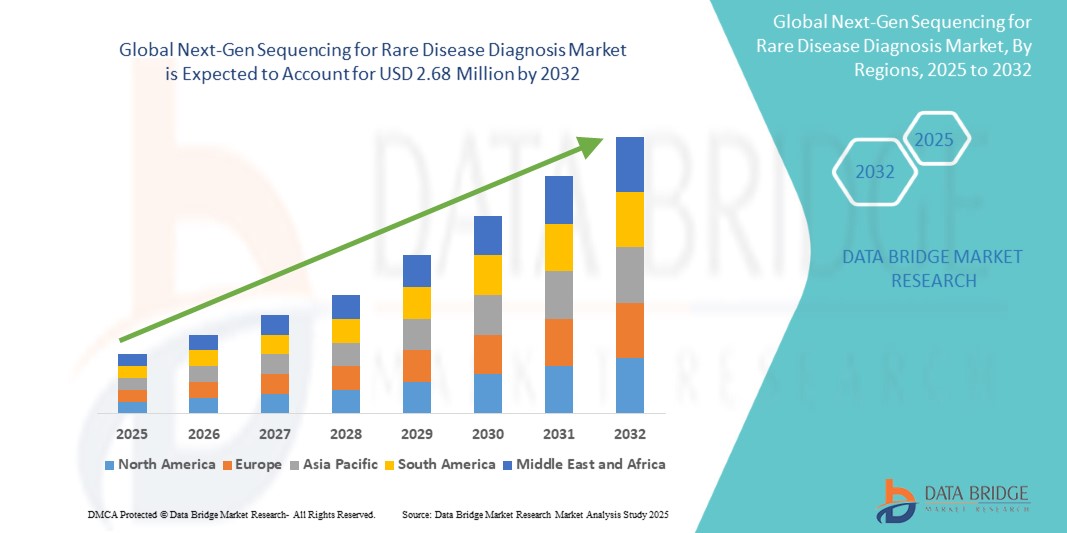
Global next-gen sequencing for rare disease diagnosis market size was valued at USD 1.44 million in 2024 and is projected to reach USD 2.68 million by 2032, with a CAGR of 8.1% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Next-Gen Sequencing for Rare Disease Diagnosis Market Trends
“Increased Adoption of NGS for Early Diagnosis”
The adoption of next-generation sequencing (NGS) technologies, such as Whole Genome Sequencing (WGS) and Whole Exome Sequencing (WES), is increasingly focused on the early diagnosis of rare genetic diseases. These technologies enable precise identification of genetic mutations at a much earlier stage than traditional methods, allowing for timely diagnosis of conditions that would otherwise be difficult to detect. This trend is leading to more accurate and personalized treatment plans for patients, improving clinical outcomes by targeting the specific genetic causes of diseases. As NGS technologies continue to advance, healthcare providers are integrating them more widely into diagnostic workflows, aiming to enhance the accuracy and efficiency of diagnosing rare diseases from an early stage.
Report Scope and Next-Gen Sequencing for Rare Disease Diagnosis Market Segmentation
|
Attributes |
Next-Gen Sequencing for Rare Disease Diagnosis Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
Illumina, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), Pacific Biosciences (U.S.), Oxford Nanopore Technologies (U.K.), BGI Genomics (China), Agilent Technologies, Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), PerkinElmer, Inc. (U.S.), OPKO Health (U.S.), Twist Bioscience (U.S.), Bio-Rad Laboratories, Inc. (U.S.), QIAgen N.V. (Germany), Mammoth Biosciences (U.S.), New England Biolabs, Inc. (U.S.), SomaLogic, Inc. (U.S.), and Nanostring Technologies, Inc. (U.S.). |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Next-Gen Sequencing for Rare Disease Diagnosis Market Definition
Next-generation sequencing (NGS) for rare disease diagnosis refers to advanced genomic technologies used to rapidly sequence DNA or RNA to identify genetic mutations associated with rare diseases. NGS allows for the simultaneous analysis of millions of DNA or RNA fragments, providing a comprehensive view of the genome or specific genes linked to rare genetic disorders. This method enables accurate, early diagnosis of conditions that may be difficult to detect with traditional techniques, offering insights into genetic variations, mutations, and disease mechanisms. NGS plays a crucial role in personalized medicine, helping clinicians tailor treatment plans based on the specific genetic makeup of patients with rare diseases.
Next-Gen Sequencing for Rare Disease Diagnosis Market Dynamics
Drivers
- Growing Prevalence of Rare Diseases
The growing prevalence of rare diseases is a significant driver for the next-gen sequencing (NGS) for rare disease diagnosis market. As awareness of rare genetic disorders increases globally, more patients are being diagnosed with conditions that were once challenging to identify through traditional diagnostic methods. NGS technologies, such as Whole Genome Sequencing (WGS) and Whole Exome Sequencing (WES), allow for faster and more accurate detection of genetic mutations, facilitating early and precise diagnoses. These technologies enable the identification of even the most complex genetic variations, which is critical for diseases with heterogeneous symptoms and limited treatment options. As healthcare systems and research institutions focus more on rare diseases, NGS is becoming an essential tool in identifying genetic causes and informing personalized treatment plans. The rise in awareness, combined with improved NGS accessibility and affordability, is expected to continue driving the market as more individuals seek accurate diagnosis and targeted therapies. For Instance, in July 2024, according to an article published by Elsevier Ltd., rare diseases impact more than 300 million people globally and are increasingly being recognized as a critical health priority. This growing awareness is expected to drive the demand for advanced diagnostic solutions, such as Next-Gen Sequencing, to improve early detection and treatment of rare conditions.
- Advancements in NGS Technology
Advancements in next-gen sequencing (NGS) technology is a key driver in the rare disease diagnosis market. Over the years, NGS platforms have seen significant improvements in accuracy, speed, and cost-efficiency, making genetic testing more reliable and accessible. The enhanced sequencing accuracy allows for the detection of even the smallest genetic mutations, providing detailed insights into rare genetic disorders that might have been missed by traditional methods. Moreover, advancements in sequencing speed have dramatically reduced turnaround times, enabling quicker diagnoses and more timely interventions. Additionally, the reduction in the cost of NGS has made it more feasible for a broader range of healthcare providers and patients, especially in regions with emerging economies. As these technological advancements continue, NGS is becoming an increasingly vital tool in the diagnosis of rare diseases, providing more comprehensive and precise genetic testing for personalized treatments and better patient outcomes. In July 2023, according to an article published by National Institutes of Health, Third-generation sequencing technologies are the latest in DNA sequencing, offering solutions to previous limitations. They allow long-read sequencing, enabling the sequencing of much larger DNA fragments compared to earlier methods. Instances include PacBio Sequencing, which uses single-molecule, real-time (SMRT) technology for long-read sequencing, and Oxford Nanopore sequencing, which uses nanopore technology for portable, long-read sequencing with real-time analysis.
Opportunities
- Increasing Collaborations and Partnerships
Increased collaborations and partnerships between biotechnology firms, research institutes, and healthcare providers present a significant opportunity for the next-gen sequencing (NGS) for Rare disease diagnosis market. By working together, these organizations can combine their expertise in genomics, diagnostics, and healthcare to accelerate the development of innovative NGS-based diagnostic solutions. Such collaborations enable the sharing of resources, knowledge, and technologies, which can help improve the accuracy, speed, and accessibility of genetic testing for rare diseases. Research institutes contribute valuable insights into disease mechanisms and genetic markers, while biotechnology firms offer advanced sequencing platforms, and healthcare providers facilitate the clinical application of these technologies. These partnerships can also help overcome the challenges related to the high cost of NGS, making it more accessible to a broader range of patients globally. By strengthening these collaborations, the development of novel diagnostic solutions can be expedited, improving the early detection and personalized treatment of rare genetic diseases. For Instance, in September 2024, according to an article published by MGI Tech Co., Ltd., MGI Tech Co., Ltd. has formed a strategic partnership with Dasa to enhance access to next-generation genomics for Brazilian patients and drive progress in Brazil's healthcare sector. This collaboration is expected to open new opportunities for MGI Tech by expanding its reach in the Brazilian market and advancing the adoption of advanced genomic technologies in the region.
- Integration with Artificial Intelligence (AI) and Machine Learning (ML)
The integration of Artificial Intelligence (AI) and Machine Learning (ML) with next-gen sequencing (NGS) technology holds significant potential in advancing the diagnosis of rare diseases. AI and ML algorithms can process and analyze vast amounts of genetic data much more efficiently than traditional methods. These technologies can identify complex genetic mutations and disease patterns that may not be immediately obvious, leading to more accurate diagnoses. AI-driven tools can also learn from large datasets, continuously improving their ability to interpret genetic variations, which enhances the precision of NGS-based diagnostics over time. Additionally, AI and ML can help in personalizing treatment strategies by predicting how patients will respond to specific therapies based on their genetic makeup. This integration can significantly increase diagnostic efficiency, reduce errors, and help clinicians design targeted treatments for rare diseases, ultimately improving patient outcomes and reducing healthcare costs by minimizing trial-and-error approaches in therapy.
Restraints/Challenges
- High Cost of NGS Technology
The high cost of next-gen sequencing (NGS) technology is a major restraint in the NGS for rare disease diagnosis market. While NGS offers highly accurate and efficient diagnostic capabilities, the initial investment required for sequencing platforms is substantial. Additionally, ongoing expenses for reagents, consumables, and maintenance of the equipment can make it difficult for healthcare providers, especially those in resource-limited settings, to afford these technologies. The costs associated with data analysis and interpretation also contribute to the financial burden, limiting accessibility in smaller healthcare facilities or regions with constrained healthcare budgets. This can result in unequal access to advanced diagnostic tools for rare diseases, particularly in emerging markets. As a result, the widespread adoption of NGS may be slowed, as healthcare systems and providers may prioritize more affordable, traditional diagnostic methods. This financial barrier poses a challenge to the overall growth of the NGS market for rare disease diagnosis. According to an article published by Genohub Inc., the high cost of NGS platforms, such as Illumina GAII X (USD 256,000) and PacBio RS (USD 695,000), underscores the financial barrier to widespread adoption. These significant upfront costs, along with ongoing maintenance and consumables, limit access, especially for smaller healthcare providers and resource-limited regions, acting as a restraint on the NGS market's growth.
- Data Interpretation and Analysis Complexity
Data interpretation and analysis complexity presents a significant challenge in the next-gen sequencing (NGS) for Rare Disease Diagnosis Market. NGS generates vast amounts of genetic data, which, while powerful, can be difficult to analyze and interpret, particularly for rare diseases where the genetic mutations involved may not be well understood. Identifying clinically relevant variants requires sophisticated bioinformatics tools and expert knowledge, as distinguishing between pathogenic mutations and benign genetic variations can be a complex task. Even with advanced algorithms, the potential for misinterpretation remains, which can lead to incorrect diagnoses or inappropriate treatment decisions. Moreover, the accuracy of diagnosis heavily relies on the quality of the data analysis, making it essential for skilled professionals to oversee this process. The requirement for highly trained personnel adds to the cost and resource demands, hindering the widespread adoption of NGS for rare disease diagnosis, especially in regions with limited access to specialized expertise.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Next-Gen Sequencing for Rare Disease Diagnosis Market Scope
The market is segmented on the basis of product type, technology, application, and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Reagents & Consumables
- Instruments
- Software
Technology
- Whole Genome Sequencing (WGS)
- Whole Exome Sequencing (WES)
- Targeted Sequencing
- RNA Sequencing
Application
- Rare Genetic Diseases
- Cancer
- Neurological Disorders
- Infectious Diseases
End-User
- Hospitals
- Diagnostic Laboratories
- Research Institutes
- Pharmaceutical & Biotech Companies
Next-Gen Sequencing for Rare Disease Diagnosis Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, product type, technology, application, and end-user as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to its highly developed healthcare infrastructure, which facilitates the widespread adoption of advanced diagnostic technologies. The region’s robust research initiatives, driven by significant investments in innovation and medical research, further strengthen its position in the market.
Asia-Pacific is expected to be the fastest growing due to increasing investments in healthcare infrastructure and medical technologies. As emerging economies in the region prioritize improving healthcare access and quality, the demand for efficient and affordable diagnostic solutions is rising.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Next-Gen Sequencing for Rare Disease Diagnosis Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Next-Gen Sequencing for Rare Disease Diagnosis Market Leaders Operating in the Market Are:
- Illumina, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Pacific Biosciences (U.S.)
- Oxford Nanopore Technologies (U.K.)
- BGI Genomics (China)
- Agilent Technologies, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- PerkinElmer, Inc. (U.S.)
- OPKO Health (U.S.)
- Twist Bioscience (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- QIAgen N.V. (Germany)
- Mammoth Biosciences (U.S.)
- New England Biolabs, Inc. (U.S.)
- SomaLogic, Inc. (U.S.)
- Nanostring Technologies, Inc. (U.S.)
Latest Developments in Next-Gen Sequencing for Rare Disease Diagnosis Market
- In October 2024, Illumina, Inc. launched its MiSeq i100 Series of sequencing systems, offering unmatched benchtop speed and ease of use to enhance next-generation sequencing (NGS) in laboratories. This innovation will help Illumina strengthen its leadership in the NGS market by providing more efficient, user-friendly solutions for a wider range of customers
- In September 2024, at the Illumina India Genomics Summit, Illumina unveiled the creation of a Global Capability Center in Bengaluru, India, aimed at expanding its technology workforce to better serve its global customer base. This expansion will enhance the company’s innovation and support capabilities worldwide
- In September 2024, MGI Tech Co., Ltd. has formed a strategic partnership with Dasa to enhance Brazilian patients' access to next-generation genomics and drive significant progress in Brazil's healthcare sector. This collaboration will strengthen MGI Tech's presence in the Brazilian market and accelerate the adoption of advanced genomic technologies
- In August 2024, Ambry Genetics introduced the ExomeReveal test, a multiomic exome sequencing tool designed to enhance rare disease detection beyond traditional DNA-based methods. By incorporating its expertise in RNA analysis from hereditary cancer testing, the ExomeReveal test improves diagnostic yield for rare diseases. This advancement will help Ambry Genetics expand its diagnostic capabilities and strengthen its position in the rare disease market
- In April 2024, GeneDx has announced a strategic partnership with Komodo Health to expand access to its de-identified rare disease data set, now available through Komodo Health’s MapEnhance offering, which includes data from over 500,000 exomes. This collaboration will provide biopharma companies with valuable genetic insights, enhancing drug pipeline development and clinical trial enrollment. The partnership will bolster GeneDx's position in the biopharma sector by facilitating faster drug development and broader industry collaboration
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.