Global Non Muscle Invasive Bladder Cancer Market
Market Size in USD Billion
CAGR :
% 
 USD
14.00 Billion
USD
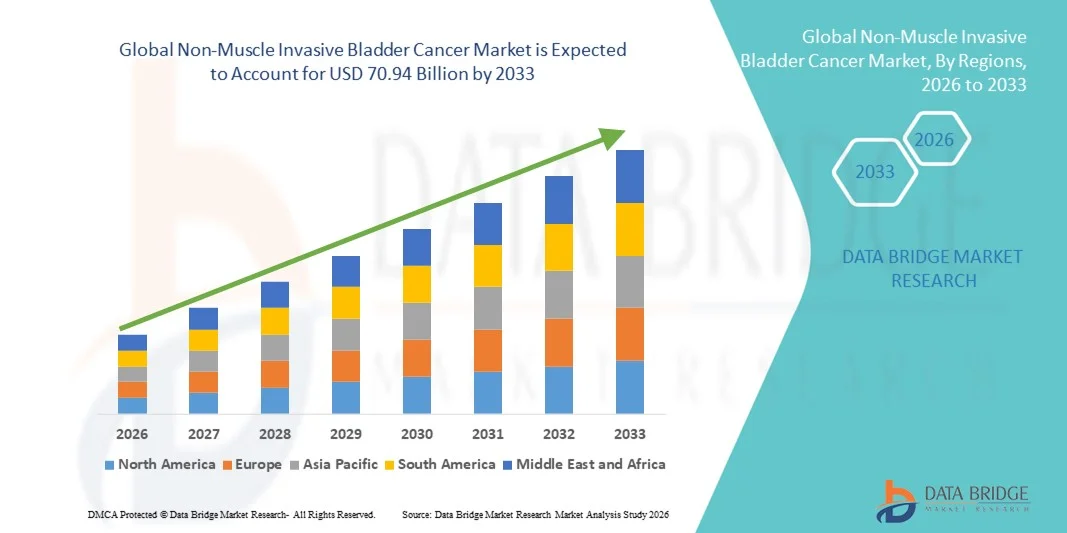
70.94 Billion
2025
2033
USD
14.00 Billion
USD
70.94 Billion
2025
2033
| 2026 –2033 | |
| USD 14.00 Billion | |
| USD 70.94 Billion | |
|
|
|
|
Non-Muscle Invasive Bladder Cancer Market Size
- The global non-muscle invasive bladder cancer market size was valued at USD 14.00 billion in 2025 and is expected to reach USD 70.94 billion by 2033, at a CAGR of 22.49% during the forecast period
- The market growth is largely fueled by the increasing prevalence of bladder cancer globally and the growing adoption of minimally invasive treatment options, leading to higher demand for advanced diagnostic and therapeutic solutions. Rising awareness of early cancer detection and the growing use of targeted therapies and immunotherapy are further supporting market expansion
- Furthermore, advancements in diagnostic imaging, molecular testing, and drug development are establishing Non-Muscle Invasive Bladder Cancer (NMIBC) treatments as a vital focus area in oncology. These converging factors are accelerating the uptake of NMIBC solutions, thereby significantly boosting the industry's growth
Non-Muscle Invasive Bladder Cancer Market Analysis
- Non-Muscle Invasive Bladder Cancer (NMIBC), representing the majority of bladder cancer cases, is increasingly recognized as a critical public health concern due to its high recurrence rates and substantial treatment costs. The market is witnessing robust growth driven by the rising prevalence of bladder cancer, the aging global population, and advancements in diagnostic and therapeutic technologies
- The escalating demand for NMIBC treatments is primarily fueled by the growing adoption of immunotherapies, targeted drugs, and minimally invasive surgical techniques. In addition, the development of innovative intravesical therapies and molecular diagnostics for early detection and monitoring is enhancing patient outcomes and expanding the market landscape
- North America dominated the non-muscle invasive bladder cancer (NMIBC) market with the largest revenue share of 41.2% in 2025, driven by well-established healthcare infrastructure, high adoption of intravesical therapies such as BCG and gemcitabine, and strong presence of leading pharmaceutical manufacturers like Merck & Co. and Pfizer. The region also benefits from robust clinical research programs and favorable reimbursement policies that promote early diagnosis and treatment
- Asia-Pacific is expected to be the fastest-growing region in the non-muscle invasive bladder cancer market during the forecast period, registering a CAGR from 2026 to 2033, driven by improving healthcare access, increasing awareness of urological diseases, and rising healthcare expenditures in countries such as China, India, and Japan. Growing adoption of advanced diagnostic tools and government initiatives for cancer control are further accelerating regional market growth
- The High-Grade Tumours segment dominated with a market share of 57.9% in 2025, due to their high risk of recurrence and progression to muscle-invasive disease
Report Scope and Non-Muscle Invasive Bladder Cancer Market Segmentation
|
Attributes |
Non-Muscle Invasive Bladder Cancer Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Non-Muscle Invasive Bladder Cancer Market Trends
Enhanced Diagnostic Precision Through AI and Imaging Integration
- A significant and accelerating trend in the global Non-Muscle Invasive Bladder Cancer (NMIBC) market is the deepening integration of artificial intelligence (AI), advanced imaging modalities, and digital diagnostic platforms, which is revolutionizing early cancer detection and treatment planning. This convergence of technologies is enhancing diagnostic accuracy, reducing recurrence rates, and improving patient outcomes
- For instance, in February 2025, Olympus Corporation launched an AI-powered cystoscopy system capable of real-time lesion detection, helping urologists identify flat or small papillary tumors that are often missed during conventional cystoscopy. Similarly, Karl Storz SE & Co. KG introduced AI-assisted video cystoscopes that automatically highlight suspicious mucosal areas, improving diagnostic precision and biopsy targeting
- AI integration in NMIBC diagnostics allows continuous learning from vast imaging datasets, enabling predictive modeling for recurrence risk and disease progression. For instance, Boston Scientific’s AI-driven visualization software enhances image contrast and identifies abnormal vascular patterns that may indicate malignancy. Furthermore, AI-based pathology platforms are improving the speed and accuracy of urine cytology interpretations, reducing diagnostic variability
- The seamless integration of AI with blue-light cystoscopy (BLC) and narrow-band imaging (NBI) technologies allows clinicians to visualize tumor margins more effectively, facilitating complete tumor resections during transurethral resection of bladder tumors (TURBT)
- This trend toward more intelligent, data-driven, and image-enhanced diagnostic systems is fundamentally reshaping how NMIBC is detected, monitored, and managed. Consequently, companies such as Photocure ASA and Olympus are investing heavily in AI-enabled diagnostic innovations that support personalized treatment decisions and continuous disease surveillance
- The demand for AI- and imaging-integrated diagnostic systems is growing rapidly across hospitals and urology centers, as clinicians increasingly prioritize precision, speed, and minimally invasive approaches to bladder cancer management
Non-Muscle Invasive Bladder Cancer Market Dynamics
Driver
Rising Disease Prevalence and Advancements in Diagnostic and Therapeutic Technologies
- The growing global burden of bladder cancer, especially among aging populations and those with exposure to smoking and occupational carcinogens, is driving significant demand for early detection and effective treatment solutions in the NMIBC market
- For instance, in April 2025, Ferring Pharmaceuticals and FerGene Inc. announced promising results from their phase III clinical trial for nadofaragene firadenovec (Adstiladrin), a novel gene therapy that demonstrated durable responses in BCG-unresponsive NMIBC patients. Such developments highlight a strong shift toward next-generation, targeted therapeutic solutions
- As healthcare systems increasingly prioritize precision oncology, advances in diagnostic imaging, biomarker-based testing, and immunotherapy are driving adoption across hospitals and research centers. Innovations such as AI-assisted cystoscopy and next-generation sequencing (NGS)-based diagnostic assays are improving disease staging and recurrence monitoring
- Furthermore, the growing investments in urology-focused research and the development of combination treatment regimens that integrate intravesical therapies with immuno-oncology agents are strengthening the NMIBC treatment landscape
- The rising emphasis on early screening programs, the expansion of healthcare infrastructure in emerging economies, and the availability of minimally invasive diagnostic tools are further propelling market growth
- Overall, the NMIBC market continues to expand due to a combination of technological progress, therapeutic innovation, and increasing awareness regarding the importance of early diagnosis and recurrence prevention
Restraint/Challenge
High Treatment Costs, Recurrence Rates, and Limited Access to Advanced Diagnostic Tools
- Despite technological advancements, the NMIBC market faces major challenges related to high recurrence rates, costly treatment procedures, and limited access to advanced diagnostic systems, particularly in low- and middle-income countries
- For instance, NMIBC patients often require lifelong surveillance through cystoscopy and urine cytology, which can be expensive and burdensome for healthcare systems. Studies indicate that bladder cancer has one of the highest lifetime treatment costs per patient among all cancers due to frequent follow-ups and recurring interventions
- Addressing these challenges requires improving affordability and accessibility of blue-light cystoscopy, molecular diagnostics, and immunotherapy-based treatments. Companies such as Olympus and Photocure are focusing on cost-effective imaging innovations to support broader clinical adoption
- In addition, limited patient awareness and delayed diagnosis remain barriers to early-stage detection in developing regions, where bladder cancer screening programs are often lacking
- The recurrence rate of NMIBC, which can exceed 50–70% within five years, underscores the urgent need for continuous innovation in surveillance technologies and personalized treatment strategies
- Overcoming these challenges through reimbursement support, public screening initiatives, and greater clinical training in advanced diagnostic tools will be essential for sustaining long-term growth in the NMIBC market
Non-Muscle Invasive Bladder Cancer Market Scope
The market is segmented on the basis of stage, treatment class, malignant potential, and end-user.
- By Stage
On the basis of stage, the Non-Muscle Invasive Bladder Cancer (NMIBC) market is segmented into Ta, Tis, and T1. The Ta segment dominated the largest market revenue share of 46.8% in 2025, driven by its high prevalence and early detection rates. These tumors are confined to the bladder lining, making them suitable for early interventions such as transurethral resection of bladder tumor (TURBT) combined with adjuvant intravesical therapy. The segment benefits from improved diagnostic tools including blue light cystoscopy and narrow-band imaging, which enhance tumor visualization and accuracy of diagnosis. Strong patient awareness and routine screening programs increase detection rates, contributing to dominance. Favorable reimbursement frameworks and growing investments in urology care strengthen adoption. Its relatively lower recurrence risk compared to T1 tumors enhances preference among clinicians. The segment also sees strong hospital-based adoption due to integrated care pathways. Government initiatives promoting early-stage bladder cancer detection further reinforce market leadership. Multidisciplinary management and standardized treatment protocols ensure robust clinical outcomes. Continuous R&D in optimizing adjuvant therapies sustains revenue dominance.
The T1 segment is projected to witness the fastest CAGR of 9.9% from 2026 to 2033, fueled by increasing diagnosis of lamina propria-invasive tumors requiring more aggressive treatment. Growing adoption of immunotherapy combinations and early intervention strategies drives the growth. Clinical trials targeting high-risk T1 patients further expand this segment’s market potential. Rising awareness of recurrence risks and progression to muscle-invasive disease supports adoption. Technological advances in imaging and surveillance enhance detection. Hospitals and specialized clinics are increasingly using minimally invasive procedures for T1 tumors. Patient education programs on follow-up care contribute to segment expansion. Early adoption of novel therapies in developed countries accelerates growth. Regulatory approvals for new T1 treatments support revenue generation. Enhanced clinical guidelines focusing on high-risk tumors stimulate adoption globally. Increasing prevalence of T1 cases among aging populations provides sustained growth.
- By Treatment Class
On the basis of treatment class, the NMIBC market is segmented into Chemotherapy, Immunotherapy, Surgery, Radiation Therapy, and Intravesical Therapy. The Immunotherapy segment dominated the market with a revenue share of 40.2% in 2025, led by widespread use of BCG therapy and checkpoint inhibitors like pembrolizumab for BCG-unresponsive patients. Its targeted mechanism and durable response make it a preferred treatment. Regulatory approvals and adoption in standard treatment protocols drive growth. High clinical efficacy and ability to reduce recurrence risk contribute to leadership. Hospitals and oncology centers prioritize immunotherapy for high-risk NMIBC. Strong ongoing R&D and clinical trial pipelines support segment dominance. Integration with personalized medicine approaches enhances adoption. Adoption in developed and emerging markets is rising. Reimbursement support for immunotherapies accelerates revenue growth. Growing incidence of recurrent NMIBC ensures steady patient pool. Physician preference for immunotherapy over systemic chemotherapy sustains dominance. Clinical guidelines emphasizing BCG therapy in NMIBC treatment support segment stability.
The Intravesical Therapy segment is anticipated to witness the fastest CAGR of 10.5% from 2026 to 2033, driven by innovations in localized therapy delivery, including gene therapy approaches such as nadofaragene firadenovec. Preference for minimally invasive treatments reduces systemic side effects, increasing patient compliance. Rising adoption in outpatient and specialized clinics fuels growth. Development of cost-effective delivery systems enhances accessibility. Clinical trials demonstrating improved outcomes boost adoption. Increasing patient awareness regarding reduced recurrence risk supports growth. Growing collaborations between biotech firms and hospitals drive segment expansion. Regulatory incentives for advanced intravesical therapies support faster adoption. Expansion of treatment indications increases patient eligibility. Rising use in combination with BCG or chemotherapy enhances effectiveness. Continuous improvements in treatment precision accelerate uptake. Increasing focus on reducing hospital stay and procedure costs fuels market momentum.
- By Malignant Potential
On the basis of malignant potential, the NMIBC market is segmented into Low-Grade Tumours and High-Grade Tumours. The High-Grade Tumours segment dominated with a market share of 57.9% in 2025, due to their high risk of recurrence and progression to muscle-invasive disease. These tumors often require TURBT followed by adjuvant BCG therapy or systemic treatment in certain cases. Strong adoption is observed in hospitals and specialized clinics. Higher clinical monitoring requirements sustain revenue generation. Regulatory approvals and incorporation into clinical guidelines support segment dominance. Ongoing R&D in immunotherapies targets high-grade tumor management. Advanced diagnostic tools improve early identification, enhancing market uptake. Growing aging population increases high-grade tumor prevalence. High-grade tumors attract attention for personalized medicine approaches. Rising awareness of aggressive NMIBC types supports early intervention adoption. Increasing government initiatives for bladder cancer treatment reinforce dominance. Continuous innovation in therapeutic strategies ensures sustained growth.
The Low-Grade Tumours segment is projected to witness the fastest CAGR of 8.8% from 2026 to 2033, driven by enhanced early detection through urine-based molecular diagnostics and widespread screening programs. Focus on minimally invasive management reduces patient burden. Increasing adoption in outpatient clinics supports growth. Development of cost-effective surveillance techniques enhances accessibility. Rising awareness among patients and caregivers accelerates adoption. Technological advances in imaging improve accuracy. Integration with personalized monitoring platforms boosts efficacy. Early intervention strategies reduce long-term healthcare costs. Clinical evidence supports conservative treatment adoption. Growth in emerging markets due to improved healthcare access fuels expansion. Collaboration between diagnostics firms and hospitals accelerates segment uptake. Regulatory approvals for novel low-grade therapies enhance adoption.
- By End-User
On the basis of end-user, the NMIBC market is segmented into Hospitals, Specialized Clinics, and Others. The Hospitals segment dominated with a revenue share of 62.8% in 2025, attributed to comprehensive infrastructure, availability of multidisciplinary care teams, and access to advanced diagnostics and therapeutics. Hospitals provide integrated treatment and monitoring pathways, enhancing patient outcomes. Investments in oncology departments and high hospitalization rates for intravesical therapy further reinforce dominance. Hospitals benefit from greater regulatory compliance and standardized treatment protocols. Strong presence of key equipment suppliers and therapy providers supports market leadership. Established referral networks drive patient influx to hospitals. Hospitals have better access to skilled clinicians for high-grade tumor management. Advanced imaging and surgical technologies in hospitals increase adoption. Reimbursement frameworks favor hospital-based interventions. Government and insurance support sustains hospital dominance. Hospitals’ capability for continuous monitoring ensures patient follow-up adherence.
The Specialized Clinics segment is expected to witness the fastest CAGR of 9.7% from 2026 to 2033, driven by growing outpatient care adoption, personalized treatment models, and minimally invasive procedure availability. Rising demand for cost-effective and patient-centric care supports growth. Adoption of flexible cystoscopes and intravesical therapies in clinics accelerates expansion. Specialized clinics cater to recurrent NMIBC cases efficiently. Collaborations with hospitals and biotech firms expand service offerings. Increasing awareness of targeted therapies drives patient preference. Early detection programs boost clinic-based treatments. Lower operational costs compared to hospitals enhance adoption. Expanding healthcare insurance coverage supports outpatient procedures. Technological advancements enable clinics to deliver high-quality care. Growing population and urbanization in emerging markets propel market growth. Government initiatives promoting clinic-based early detection enhance penetration.
Non-Muscle Invasive Bladder Cancer Market Regional Analysis
- North America dominated the non-muscle invasive bladder cancer (NMIBC) market with the largest revenue share of 41.2% in 2025, driven by a well-established healthcare infrastructure, early diagnosis rates, and strong availability of intravesical therapies such as Bacillus Calmette–Guérin (BCG) and gemcitabine
- The region’s dominance is also attributed to significant investments in clinical research and the presence of leading pharmaceutical players such as Merck & Co., Pfizer Inc., and Johnson & Johnson actively developing and marketing NMIBC therapeutics
- In addition, favorable reimbursement frameworks and rising awareness about bladder cancer screening contribute to high treatment adoption rates across hospitals and urology clinics
U.S. Non-Muscle Invasive Bladder Cancer Market Insight
The U.S. non-muscle invasive bladder cancer (NMIBC) market accounted the total North American NMIBC market share in 2025, driven by widespread use of BCG immunotherapy and increasing adoption of maintenance treatments for recurrence prevention. Strong clinical trial activity supported by the National Cancer Institute (NCI) and growing collaborations between biotech startups and academic institutions continue to accelerate innovation in NMIBC treatment. High healthcare spending and improved access to advanced diagnostic methods such as cystoscopy and urine-based biomarkers further enhance market growth in the country.
Europe Non-Muscle Invasive Bladder Cancer Market Insight
Europe non-muscle invasive bladder cancer (NMIBC) market held a notable share of the global NMIBC market in 2025, supported by strong regulatory support for novel drug approvals and increasing adoption of intravesical chemotherapy and immunotherapy. The region benefits from advanced healthcare systems and a growing number of cancer awareness programs that encourage early-stage diagnosis. Countries such as Germany, France, and the U.K. are witnessing increasing clinical trials evaluating combination therapies for high-risk NMIBC patients, fostering steady market expansion.
U.K. Non-Muscle Invasive Bladder Cancer Market Insight
The U.K. non-muscle invasive bladder cancer (NMIBC) market is expanding significantly due to increasing government focus on cancer control programs and the growing burden of urological disorders. Enhanced diagnostic capabilities and the integration of precision medicine in oncology are contributing to improved patient outcomes. Public healthcare support under the NHS for intravesical therapies and novel treatment pathways is further boosting treatment adoption rates.
Germany Non-Muscle Invasive Bladder Cancer Market Insight
Germany non-muscle invasive bladder cancer (NMIBC) market represents one of the key markets in Europe, supported by high healthcare spending and continuous innovation in bladder cancer therapeutics. The increasing use of intravesical chemotherapy combinations and the availability of advanced diagnostic imaging are enhancing treatment efficacy. Moreover, Germany’s strong biotechnology ecosystem and active participation in multinational clinical trials continue to drive the development of new NMIBC therapies.
Asia-Pacific Non-Muscle Invasive Bladder Cancer Market Insight
Asia-Pacific non-muscle invasive bladder cancer (NMIBC) market is projected to be the fastest-growing region, with an expected growth rate from 2026 to 2033, driven by expanding healthcare access, increased awareness of bladder cancer, and rising healthcare spending in countries such as China, India, and Japan. The region is witnessing a steady rise in diagnostic testing rates, supported by government initiatives for cancer control and private sector investment in oncology research. Improved infrastructure for hospital-based and outpatient urology care is expected to significantly expand treatment availability and affordability.
Japan Non-Muscle Invasive Bladder Cancer Market Insight
The Japanese non-muscle invasive bladder cancer (NMIBC) market is growing steadily due to a well-developed healthcare system, early screening programs, and strong emphasis on preventive oncology. The country’s aging population and high bladder cancer prevalence are driving demand for effective intravesical and immunotherapeutic treatments. Collaborations between pharmaceutical companies and academic institutions are accelerating research on biomarkers and personalized NMIBC therapies.
China Non-Muscle Invasive Bladder Cancer Market Insight
China non-muscle invasive bladder cancer (NMIBC) market held the largest NMIBC market share within Asia-Pacific in 2025, supported by significant investments in biotechnology, improved access to advanced diagnostic technologies, and a large patient population. Strong domestic manufacturing capacity and favorable government support for oncology drug development are further driving market expansion. The increasing number of local clinical trials, along with rising awareness about early cancer diagnosis, is expected to strengthen China’s position in the regional NMIBC landscape.
Non-Muscle Invasive Bladder Cancer Market Share
The Non-Muscle Invasive Bladder Cancer industry is primarily led by well-established companies, including:
• F. Hoffmann-La Roche Ltd (Switzerland)
• Merck & Co., Inc. (U.S.)
• Bristol Myers Squibb (U.S.)
• Pfizer Inc. (U.S.)
• Astellas Pharma Inc. (Japan)
• Eisai Co., Ltd. (Japan)
• Johnson & Johnson (U.S.)
• Novartis AG (Switzerland)
• Spectrum Pharmaceuticals, Inc. (U.S.)
• VAXIMM AG (Switzerland)
• Dr. Reddy’s Laboratories Ltd. (India)
• Asieris Pharmaceuticals (China)
• TheraBio (U.S.)
• Anchiano Therapeutics (Israel)
• Ferring Pharmaceuticals (Switzerland)
• ImmunityBio, Inc. (U.S.)
• Endo International plc (Ireland)
• IBSA Institut Biochimique SA (Switzerland)
• OncoTherapy Science, Inc. (Japan)
• Steba Biotech (Luxembourg)
Latest Developments in Global Non-Muscle Invasive Bladder Cancer Market
- In December 2022, the Nadofaragene firadenovec‑vncg gene therapy (brand name Adstiladrin) was approved by the U.S. Food & Drug Administration for adult patients with BCG‑unresponsive non‑muscle‑invasive bladder cancer (with carcinoma in situ and/or papillary tumors)
- In April 2024, the FDA approved the first‑in‑class IL‑15 receptor agonist therapy Nogapendekin alfa inbakicept‑pmln (brand name Anktiva) in combination with BCG for adult patients with BCG‑unresponsive NMIBC with carcinoma in situ (CIS) with or without papillary tumors
- In June 2025, the FDA approved the intravesical solution of Mitomycin intravesical solution (brand name Zusduri; formerly UGN‑102) for adult patients with recurrent low‑grade, intermediate‑risk non‑muscle‑invasive bladder cancer (LG‑IR‑NMIBC)
- In January 2025, Pfizer Inc. announced that its experimental therapy Sasanlimab in combination with BCG met the primary endpoint in a late‑stage trial in high‑risk NMIBC patients, significantly extending recurrence‑free duration when compared with BCG alone
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.