Global Noonan Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
861.98 Million
USD
1,823.69 Million
2024
2032
USD
861.98 Million
USD
1,823.69 Million
2024
2032
| 2025 –2032 | |
| USD 861.98 Million | |
| USD 1,823.69 Million | |
|
|
|
|
Noonan Syndrome Market Size
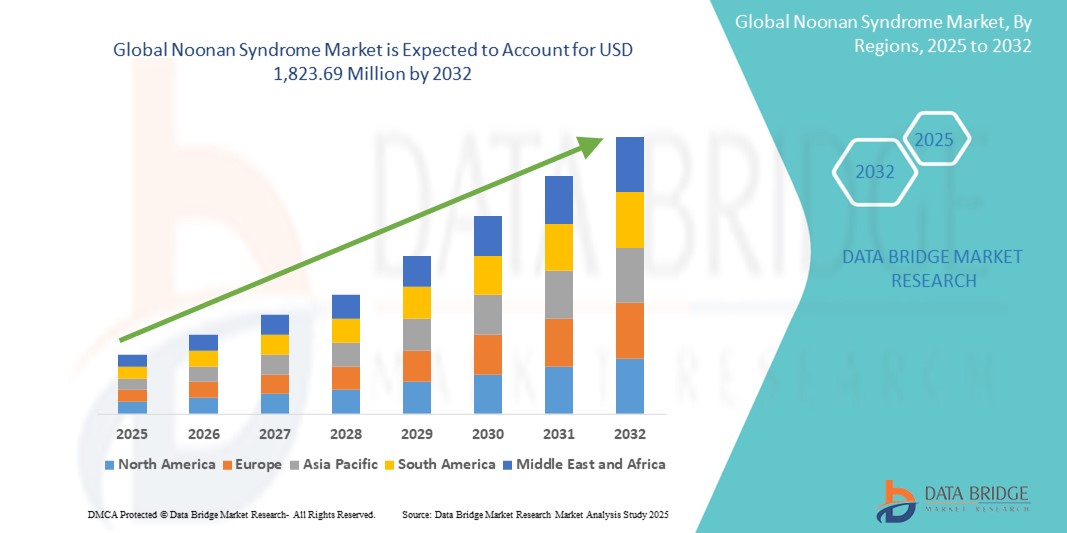
- The global Noonan syndrome market size was valued at USD 861.98 million in 2024 and is expected to reach USD 1,823.69 million by 2032, at a CAGR of 9.82% during the forecast period
- The market growth is largely fueled by increasing awareness, early diagnosis, and advancements in genetic testing and personalized medicine, which are enhancing patient outcomes and expanding treatment options for rare genetic disorders such as Noonan syndrome
- Furthermore, growing research initiatives, favorable regulatory support for orphan drugs, and rising investment in rare disease therapeutics are establishing targeted therapies as a key area of innovation. These converging factors are accelerating the development pipeline, thereby significantly boosting the industry’s growth
Noonan Syndrome Market Analysis
- Noonan syndrome, a genetic disorder characterized by distinctive facial features, heart defects, and developmental delays, is gaining growing clinical attention due to advancements in genetic diagnostics and increased awareness among healthcare providers and patients across both developed and emerging regions
- The escalating demand for targeted therapies is primarily fueled by the expansion of precision medicine, improved accessibility to molecular genetic testing, and increased focus on early diagnosis and intervention in pediatric and prenatal care settings
- North America dominated the Noonan syndrome market with the largest revenue share of 39% in 2024, driven by robust healthcare infrastructure, early adoption of genetic testing, and a supportive regulatory framework for orphan drugs, with the U.S. leading in clinical trials and approvals for targeted therapies addressing rare genetic conditions
- Asia-Pacific is expected to be the fastest growing region in the Noonan syndrome market during the forecast period due to rising healthcare investments, increasing awareness of rare genetic disorders, and improving diagnostic capabilities
- Genetic Test segment dominated the Noonan syndrome market with a market share of 43.2% in 2024, driven by its high diagnostic accuracy, early detection capability, and increased availability of targeted genetic panels
Report Scope and Noonan Syndrome Market Segmentation
|
Attributes |
Noonan Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Noonan Syndrome Market Trends
“Rising Focus on Precision Medicine and Genetic Therapies”
- A significant and accelerating trend in the global Noonan syndrome market is the growing emphasis on precision medicine and the development of targeted genetic therapies. This shift is driven by advancements in genomic sequencing technologies and deeper understanding of RAS/MAPK pathway mutations commonly associated with Noonan syndrome
- For instance, MEK inhibitors such as trametinib are being explored in clinical studies to manage complications such as hypertrophic cardiomyopathy in Noonan syndrome patients with specific gene mutations. In addition, pharmaceutical companies are investing in research initiatives to develop mutation-specific therapies that offer improved patient outcomes
- Genetic testing is being increasingly utilized not only for accurate diagnosis but also to guide personalized treatment strategies, helping clinicians select the most effective interventions for individual patients based on their genetic profile
- The growing prevalence of prenatal and early childhood genetic screening is enabling earlier diagnosis and timely intervention, which is critical in managing congenital abnormalities and developmental delays associated with Noonan syndrome.
- This trend is further supported by increased advocacy, collaboration between biotech firms and academic institutions, and government support through orphan drug policies, collectively driving innovation and development of novel therapeutics
- The demand for personalized therapies and early genetic screening is growing rapidly across healthcare systems worldwide, as stakeholders increasingly recognize the value of mutation-specific care pathways and precision medicine in rare genetic disorders such as Noonan syndrome
Noonan Syndrome Market Dynamics
Driver
“Improved Genetic Testing and Early Diagnosis Advancing Treatment Landscape”
- The increasing accessibility and precision of genetic testing technologies, coupled with heightened awareness of rare genetic disorders, is a major driver fueling the growth of the Noonan syndrome market
- For instance, the widespread adoption of next-generation sequencing (NGS) enables early and accurate detection of Noonan syndrome-related mutations, allowing healthcare professionals to intervene earlier with appropriate care plans
- As early diagnosis facilitates better management of complications such as congenital heart defects and developmental delays, genetic counseling and pediatric care services are witnessing growing demand
- In addition, rising investments in rare disease research and supportive regulatory frameworks, including orphan drug designations and fast-track approvals, are encouraging the development of targeted therapies for Noonan syndrome, further accelerating market growth
- Healthcare providers and advocacy groups are also playing a key role in expanding awareness and education, promoting timely diagnosis and care coordination across various regions
Restraint/Challenge
“Limited Treatment Options and High Diagnostic Costs in Developing Regions”
- Despite advances in diagnosis, the lack of curative treatment options and the high cost associated with genetic testing continue to restrain the global Noonan syndrome market, particularly in low- and middle-income countries
- For instance, comprehensive genetic panels and whole-exome sequencing, while available in developed nations, remain largely inaccessible in resource-limited settings due to cost, limited infrastructure, and insufficient specialist availability
- This contributes to underdiagnosis or delayed treatment in many regions, resulting in missed opportunities for early intervention and improved outcomes
- In addition, management of Noonan syndrome typically involves multidisciplinary care teams addressing cardiovascular, developmental, and hematologic complications, which can be financially and logistically burdensome for families without adequate insurance coverage
- To overcome these challenges, global efforts are needed to expand diagnostic access, subsidize testing and care, and incentivize the development of innovative therapies targeting the underlying genetic causes of Noonan syndrome
Noonan Syndrome Market Scope
The market is segmented on the basis of diagnosis, treatment, route of administration, end-users, and distribution channel.
- By Diagnosis
On the basis of diagnosis, the Noonan syndrome market is segmented into ultrasound test, genetic test, blood test, and others. The genetic test segment dominated the market with the largest market revenue share of 43.2% in 2024, driven by its high diagnostic accuracy, capability to identify specific gene mutations such as PTPN11, and increasing availability of advanced genetic panels. The integration of next-generation sequencing (NGS) technologies and broader access through pediatric and prenatal care settings has enhanced early and precise diagnosis of Noonan syndrome.
The ultrasound test segment is expected to witness the fastest growth from 2025 to 2032, as prenatal ultrasound screening becomes more widespread for early detection of physical anomalies such as increased nuchal translucency and heart defects, enabling timely referrals for genetic confirmation and intervention.
- By Treatment
On the basis of treatment, the market is segmented into heart treatment, learning disabilities treatment, low growth treatment, vision and hearing treatment, bleeding and bruising treatment, genital problem treatment, lymphatic problem treatment, and others. The heart treatment segment dominated the market with the largest share of 33.9% in 2024, due to the high prevalence of congenital heart defects in Noonan syndrome patients, such as pulmonary valve stenosis and hypertrophic cardiomyopathy. Cardiac care remains central to disease management, involving surgical procedures, medication, and lifelong monitoring.
The low growth treatment segment is anticipated to grow at the fastest rate from 2025 to 2032, supported by increased usage of recombinant human growth hormone (rhGH) therapy in pediatric patients with confirmed growth hormone deficiency or short stature associated with Noonan syndrome.
- By Route Of Administration
On the basis of route of administration, the market is segmented into oral, parenteral, and others. The oral route dominated the market with a share of 54.4% in 2024, primarily due to the widespread use of oral supplements, cardiovascular medications, and therapies addressing developmental symptoms. Ease of administration and adherence contribute to the preference for oral formulations in long-term care.
The parenteral segment is expected to grow steadily during forecast period, driven by the use of injectable growth hormones and specialized therapies such as monoclonal antibodies and targeted inhibitors for mutation-specific symptom management.
- By End User
On the basis of end-users, the market is segmented into hospitals, clinics, home healthcare, and others. The hospitals segment accounted for the largest market share of 49.6% in 2024, due to the availability of multidisciplinary diagnostic and treatment services including cardiology, genetics, and pediatrics under one facility. Hospitals are the primary sites for genetic testing, specialist consultations, and complex interventions.
The home healthcare segment is projected to grow at the fastest pace from 2025 to 2032, driven by increasing demand for remote care, telemedicine consultations, and home-based management of chronic conditions, especially in developed countries.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, direct tenders, retail sales, and others. The hospital pharmacy segment dominated the market with a share of 37.2% in 2024, due to its integral role in dispensing specialty medications, growth hormone therapies, and genetic treatment drugs directly following diagnosis in clinical settings.
The online pharmacy segment is expected to witness the fastest growth from 2025 to 2032, fueled by increased digital health adoption, ease of access to rare disease medications, home delivery convenience, and greater awareness among caregivers and patients seeking cost-effective sourcing options.
Noonan Syndrome Market Regional Analysis
- North America dominated the Noonan syndrome market with the largest revenue share of 39% in 2024, driven by robust healthcare infrastructure, early adoption of genetic testing, and a supportive regulatory framework for orphan drugs, with the U.S. leading in clinical trials and approvals for targeted therapies addressing rare genetic conditions
- Patients and healthcare providers in the region benefit from improved access to diagnostic tools, specialist care, and mutation-specific therapies supported by government incentives and insurance coverage
- This widespread diagnosis and treatment adoption is further supported by active rare disease advocacy groups, a high concentration of clinical research institutions, and favorable regulatory frameworks, positioning North America as a leader in the management and innovation of Noonan syndrome care
U.S. Noonan Syndrome Market Insight
The U.S. Noonan syndrome market captured the largest revenue share of 83% in 2024 within North America, driven by the widespread use of advanced genetic testing and a strong focus on early diagnosis and personalized treatment. The presence of leading research institutions, active clinical trials, and supportive healthcare policies—particularly for orphan diseases—further accelerate market growth. In addition, the availability of specialized pediatric care and genetic counseling services is enhancing patient management and driving treatment adoption across the country.
Europe Noonan Syndrome Market Insight
The Europe Noonan syndrome market is projected to expand at a substantial CAGR throughout the forecast period, fueled by improved diagnostic infrastructure, rising awareness of rare genetic disorders, and strong regulatory support for orphan drugs. Increasing collaboration between academic hospitals and biotech firms is fostering the development of mutation-specific therapies. With a growing focus on early detection and multi-disciplinary care, the region is witnessing increased diagnosis rates and access to advanced treatment pathways across both public and private healthcare settings.
U.K. Noonan Syndrome Market Insight
The U.K. Noonan syndrome market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by national initiatives promoting early diagnosis of rare diseases and the integration of genomic medicine into the healthcare system. The country’s investment in genetic research and access to NHS-funded testing services are key factors enhancing diagnostic precision and facilitating timely intervention. In addition, strong public awareness campaigns and support from rare disease organizations are contributing to increased diagnosis and specialist referrals.
Germany Noonan Syndrome Market Insight
The Germany Noonan syndrome market is expected to expand at a considerable CAGR during the forecast period, driven by the country’s emphasis on innovation, genetic research, and coordinated care models. Germany’s comprehensive healthcare system supports early screening, specialist treatment, and long-term management of rare diseases such as Noonan syndrome. The integration of precision diagnostics and collaborative treatment protocols across pediatric, cardiology, and endocrinology departments is improving patient outcomes and driving market growth.
Asia-Pacific Noonan Syndrome Market Insight
The Asia-Pacific Noonan syndrome market is poised to grow at the fastest CAGR of 25% during the forecast period of 2025 to 2032, driven by increasing awareness, rising birth rates, and expanding access to genetic testing in countries such as China, Japan, and India. Government-led healthcare reforms, combined with technological advancements in diagnostics and digital health infrastructure, are facilitating earlier identification and management of Noonan syndrome. The growing pediatric population and focus on improving rare disease care are key drivers across the region.
Japan Noonan Syndrome Market Insight
The Japan Noonan syndrome market is gaining momentum due to the country’s high healthcare standards, robust diagnostic capabilities, and emphasis on genetic screening. The growing adoption of precision medicine and government-backed rare disease initiatives are enhancing diagnosis and treatment options. Japan’s aging population and increasing awareness of pediatric genetic disorders are also spurring demand for comprehensive care solutions tailored to patients with complex congenital conditions such as Noonan syndrome.
India Noonan Syndrome Market Insight
The India Noonan syndrome market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to expanding healthcare access, improved diagnostic awareness, and rising demand for pediatric genetic services. With growing urbanization and investment in medical infrastructure, India is witnessing increased uptake of genetic testing in both public and private hospitals. National health initiatives, such as Ayushman Bharat and Make in India, are supporting the availability of affordable diagnostics and therapies, driving the market’s growth trajectory
Noonan Syndrome Market Share
The Noonan syndrome industry is primarily led by well-established companies, including:
- Labcorp (U.S.)
- Invitae Corporation (U.S.)
- GeneDx, LLC (U.S.)
- CENTOGENE N.V. (Germany)
- Blueprint Genetics Oy (Finland)
- Fulgent Genetics, Inc. (U.S.)
- Ambry Genetics Corporation (U.S.)
- Eurofins Scientific SE (Luxembourg)
- Sema4 OpCo, Inc. (U.S.)
- Paragon Genomics, Inc. (U.S.)
- BioMarin Pharmaceutical Inc. (U.S.)
- Novartis AG (Switzerland)
- Sanofi S.A. (France)
- Hoffmann-La Roche Ltd (Switzerland)
- Thermo Fisher Scientific Inc. (U.S.)
- QIAGEN N.V. (Netherlands)
- Agilent Technologies, Inc. (U.S.)
- PerkinElmer (U.S.)
- MedGenome Labs Ltd. (India)
- Myriad Genetics, Inc. (U.S.)
What are the Recent Developments in Global Noonan Syndrome Market?
- In May 2024, Novartis AG initiated a Phase II clinical trial evaluating the use of MEK inhibitors in pediatric patients with Noonan syndrome presenting with hypertrophic cardiomyopathy. This trial aims to explore targeted therapeutic approaches based on the underlying genetic mutations, showcasing Novartis’ continued commitment to precision medicine and rare disease innovation. The advancement reflects growing interest in developing mutation-specific treatments for syndromes involving the RAS/MAPK pathway
- In March 2024, BioMarin Pharmaceutical Inc. announced the expansion of its rare disease research program to include genetic disorders such as Noonan syndrome, focusing on developing gene-targeted therapies. The initiative is part of a broader strategic effort to address high-unmet needs within pediatric genetic conditions and aligns with BioMarin’s track record of delivering breakthrough treatments for rare diseases
- In February 2024, the U.S. National Institutes of Health (NIH) granted funding for a collaborative research program between academic institutions and biotech firms to investigate genotype-phenotype correlations in Noonan syndrome. The initiative aims to improve diagnosis, predict treatment outcomes, and facilitate the development of more effective targeted interventions, reinforcing the importance of public-private partnerships in advancing rare disease research
- In January 2024, GeneDx, a leader in genomic diagnostics, launched an enhanced Noonan syndrome genetic panel that includes newly identified mutations and offers faster turnaround times. This development supports more accurate and timely diagnosis for pediatric patients, allowing clinicians to initiate appropriate management strategies earlier and improve long-term outcomes
- In December 2023, European Medicines Agency (EMA) granted orphan drug designation to a novel investigational therapy developed by BridgeBio Pharma for the treatment of Noonan syndrome with multiple lentigines. The designation provides regulatory incentives and underscores the therapy’s potential to address a critical gap in treatment options, marking a step forward in rare disease therapeutics within the European Union
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.