Global Nucleic Acid Based Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
4.94 Billion
USD
14.38 Billion
2024
2032
USD
4.94 Billion
USD
14.38 Billion
2024
2032
| 2025 –2032 | |
| USD 4.94 Billion | |
| USD 14.38 Billion | |
|
|
|
|
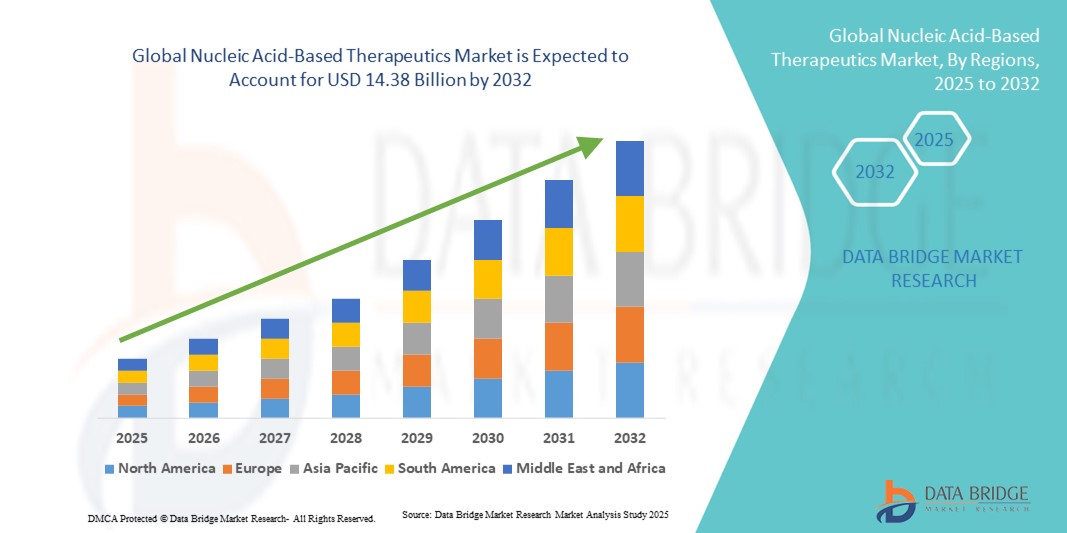
Nucleic Acid-Based Therapeutics Market Size
- The global nucleic acid-based therapeutics market size was valued at USD 4.94 billion in 2024 and is expected to reach USD 14.38 billion by 2032, at a CAGR of 14.29% during the forecast period
- The growth is primarily driven by advancements in genomic research, increased prevalence of genetic disorders, and the rising adoption of personalized medicine. These factors contribute to the development of targeted therapies that address the root causes of diseases at the molecular level
- The market's expansion is further supported by the increasing demand for precision medicine, which tailors treatment to individual genetic profiles, and the growing prevalence of rare genetic diseases, which often lack effective treatments. These trends are accelerating the adoption of nucleic acid-based therapeutics, positioning them as a cornerstone in modern medicine
Nucleic Acid-Based Therapeutics Market Analysis
- Nucleic acid-based therapeutics, including antisense oligonucleotides, siRNA, mRNA, and gene-editing therapies, are emerging as transformative solutions for treating genetic, rare, and chronic diseases, offering precise targeting of disease-causing genes at the molecular level in both clinical and research settings
- The escalating demand for nucleic acid-based therapeutics is primarily fueled by advancements in genomic research, growing prevalence of genetic disorders, and the rising adoption of personalized and precision medicine approaches that enable tailored treatment strategies
- North America dominated the nucleic acid-based therapeutics market with the largest revenue share of 42.5% in 2024, supported by well-established biotechnology infrastructure, significant R&D investments, high healthcare expenditure, and early adoption of advanced therapeutics, with the U.S. leading in clinical trials and commercialization of innovative nucleic acid therapies
- Asia-Pacific is expected to be the fastest-growing region in the nucleic acid-based therapeutics market during the forecast period due to increasing investments in biotechnology, expanding healthcare infrastructure, rising prevalence of genetic diseases, and supportive government initiatives for biopharmaceutical development
- Monogenic disorders segment dominated the nucleic acid-based therapeutics market in 2024 with a share of 57.5%, driven by the high prevalence of rare genetic diseases and the effectiveness of nucleic acid-based therapeutics in treating single-gene disorders
Report Scope and Nucleic Acid-Based Therapeutics Market Segmentation
|
Attributes |
Nucleic Acid-Based Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Nucleic Acid-Based Therapeutics Market Trends
Advancements in RNA and Gene Editing Technologies
- A major trend in the global nucleic acid-based therapeutics market is the rapid evolution of RNA-based therapies and gene-editing platforms such as CRISPR-Cas9 and siRNA technologies. These innovations are significantly enhancing the precision, safety, and efficacy of treatments for genetic and rare diseases
- For instance, recent mRNA therapies for rare genetic disorders demonstrate how targeted gene modulation can lead to significant clinical improvements. Similarly, CRISPR-based therapeutics are enabling precise correction of disease-causing mutations in monogenic disorders
- Advances in delivery technologies, such as lipid nanoparticles and viral vectors, are improving the stability and cellular uptake of nucleic acid therapeutics, increasing their therapeutic potential and patient compliance
- The integration of nucleic acid-based therapies with personalized medicine approaches allows clinicians to tailor treatment based on an individual’s genetic profile, maximizing efficacy and minimizing adverse effects
- This trend towards more precise, customizable, and advanced therapeutics is reshaping treatment paradigms for genetic diseases. Consequently, companies such as Moderna and Beam Therapeutics are developing next-generation RNA and gene-editing therapeutics targeting rare and complex genetic disorders
- The demand for nucleic acid-based therapies that are safer, more effective, and tailored to patient-specific needs is rising rapidly across both rare disease and chronic condition segments
Nucleic Acid-Based Therapeutics Market Dynamics
Driver
Increasing Prevalence of Genetic Disorders and Adoption of Precision Medicine
- The growing prevalence of genetic and rare diseases, coupled with the rising focus on precision medicine, is a key driver for the adoption of nucleic acid-based therapeutics
- For instance, the increasing number of clinical trials targeting rare monogenic disorders reflects the strong interest in developing targeted treatments that address the root cause of disease
- Patients and healthcare providers are seeking therapies that can deliver durable outcomes with fewer side effects compared to conventional treatments, which drives demand for nucleic acid-based solutions
- Furthermore, advancements in genomic research, improved diagnostic capabilities, and regulatory incentives for orphan drug development are supporting market expansion
- The ability to develop patient-specific therapies and the growing adoption of personalized medicine approaches are critical factors propelling market growth globally
- Rising investment from venture capital and pharmaceutical companies is fueling research in innovative nucleic acid technologies, expanding pipeline candidates and accelerating commercialization
- Increasing awareness among healthcare professionals and patients about the benefits of nucleic acid-based therapeutics is driving adoption across clinical and specialty treatment areas
Restraint/Challenge
Delivery Complexities and High Development Costs
- Nucleic acid-based therapeutics face challenges in safe and efficient delivery to target cells, which can limit clinical efficacy and patient adoption. Stability, immune response, and off-target effects remain critical hurdles in therapy development
- For example, achieving effective intracellular delivery without inducing toxicity continues to be a major technical challenge for siRNA and gene-editing therapeutics
- High research and development costs, coupled with complex regulatory requirements, can slow commercialization and limit accessibility, especially in developing regions
- While innovative delivery platforms such as lipid nanoparticles and viral vectors are mitigating some challenges, these solutions often increase production costs and limit scalability
- Overcoming delivery challenges, reducing development costs, and establishing clear regulatory pathways are vital for broader adoption and sustained market growth.
- Limited long-term clinical data on safety and efficacy of certain nucleic acid therapies can hinder adoption and investor confidence
- Intellectual property and patent challenges may delay development and commercialization, creating additional barriers for new entrants and smaller biotech firms
Nucleic Acid-Based Therapeutics Market Scope
The market is segmented on the basis of application, structure, technology, end-users, and distribution channel.
- By Application
On the basis of application, the nucleic acid-based therapeutics market is segmented into monogenic disorders and multigenic disorders. The monogenic disorders segment dominated the market in 2024 with a share of 57.5%, driven by the high prevalence of rare single-gene diseases and the proven effectiveness of nucleic acid therapeutics in treating these disorders. Therapies such as antisense oligonucleotides and siRNA have shown significant clinical success in correcting defective genes or modulating gene expression. Growing awareness among healthcare professionals and patients regarding the advantages of precision medicine is further boosting adoption. Favorable regulatory incentives for orphan drug development and increasing investment in rare disease research continue to support market dominance. The segment also benefits from strong clinical pipelines and collaborations between biotechnology firms and research institutions.
The multigenic disorders segment is expected to witness the fastest growth during forecast period, fueled by advancements in RNA and gene-editing technologies that allow targeted intervention in complex diseases influenced by multiple genes. Rising prevalence of chronic and lifestyle-related disorders, combined with research in polygenic therapeutic approaches, is accelerating adoption. Increasing clinical trials for multigenic conditions and the development of combination therapies also contribute to the growth trajectory. Improved diagnostics and genomic profiling facilitate the identification of patients suitable for such therapies. The trend toward personalized and precision medicine enhances the relevance of this segment.
- By Structure
On the basis of structure, the nucleic acid-based therapeutics market is segmented into single-stranded RNA/DNA and double-stranded DNA. The single-stranded RNA/DNA segment dominated the market in 2024 with a share of 62%, driven by widespread adoption of mRNA vaccines, antisense oligonucleotides, and siRNA-based therapies. Single-stranded nucleic acids allow precise targeting of mRNA and protein expression, ensuring high efficacy and lower off-target effects. Advanced delivery systems such as lipid nanoparticles improve stability and cellular uptake, increasing clinical adoption. Success in both rare and common genetic conditions further strengthens this segment. The segment also benefits from established manufacturing processes and regulatory approvals. Increasing patient awareness and physician confidence in single-stranded therapeutics reinforce market leadership.
The double-stranded DNA segment is expected to register the fastest growth during forecast period, supported by the expansion of gene therapy programs and CRISPR-based DNA editing approaches. These therapies target permanent gene correction and are increasingly explored for monogenic and complex disorders. Growth is driven by innovative vector designs and improved delivery methods. Rising R&D investments and new product pipelines further accelerate market adoption. Increased collaboration between academia and industry enables faster development. Regulatory incentives for breakthrough therapies also support rapid expansion.
- By Technology
On the basis of technology, the nucleic acid-based therapeutics market is segmented into antisense technology, gene therapy, RNA and DNA therapy, aptamers, nucleoside analogs, and others. The antisense technology segment dominated the market in 2024 with a share of 40%, owing to established clinical efficacy in treating rare genetic diseases and a strong pipeline of approved therapies. Antisense therapeutics modulate gene expression, reduce disease-causing proteins, and target specific genetic mutations effectively. Collaborations between pharmaceutical companies and research institutions enhance development. Favorable regulatory frameworks and orphan drug incentives further boost adoption. Strong clinical data and successful commercialization support continued growth. The segment remains attractive for both new and established players due to predictable therapeutic outcomes.
The gene therapy segment is expected to witness the fastest growth during forecast period, driven by advancements in viral and non-viral delivery systems and increasing focus on treating previously untreatable genetic disorders. Expanding clinical trials and growing investment in gene-editing research are key drivers. Adoption is fueled by rising awareness of long-term benefits and durable treatment outcomes. Regulatory support for breakthrough therapies enhances market potential. Collaborations between biotech firms and hospitals facilitate faster commercialization. The segment also benefits from the development of combination therapies with RNA/DNA approaches.
- By End-Users
On the basis of end-users, the nucleic acid-based therapeutics market is segmented into hospitals, research institutes, and others. The hospitals segment dominated the market in 2024 with a share of 50%, as hospitals provide clinical administration and monitoring for nucleic acid-based therapeutics. Hospitals have the infrastructure, trained personnel, and facilities for safe delivery of advanced therapies. They act as centers for patient follow-up, clinical trials, and long-term therapy management. The controlled environment ensures patient safety and treatment efficacy. Hospitals also facilitate collaboration with research institutes and pharmaceutical companies. Increasing integration of personalized medicine programs in hospitals further supports dominance.
The research institutes segment is expected to witness the fastest growth during forecast period, propelled by growing R&D activities, preclinical studies, and translational research on nucleic acid therapeutics. Research institutes often pioneer innovative technologies and therapeutic candidates. Collaborations with biotech startups and pharmaceutical companies accelerate development timelines. Increasing funding and grants support cutting-edge research. Rising focus on understanding disease mechanisms and gene function drives market adoption. Institutes also contribute to education and awareness about nucleic acid therapeutics.
- By Distribution Channel
On the basis of distribution channel, the nucleic acid-based therapeutics market is segmented into hospital pharmacy, retail pharmacy, and others. The hospital pharmacy segment dominated the market in 2024 with a share of 48%, driven by the direct administration of nucleic acid-based therapies and the need for controlled storage and handling. Hospital pharmacies ensure regulatory compliance and patient safety, which is critical for advanced therapeutics. They play a central role in dosing, monitoring, and managing patient outcomes. Hospitals often integrate pharmacy operations with clinical care and research programs. The segment benefits from established healthcare infrastructure and trained personnel. Increasing hospital-based programs for rare disease treatments further reinforce dominance.
The retail pharmacy segment is expected to witness the fastest growth during forecast period, supported by the increasing availability of home-administered therapies and growing patient preference for convenient access. Improvements in patient-friendly delivery formats, such as prefilled syringes and self-administered kits, facilitate adoption. Rising awareness of personalized medicine enhances demand. Expansion in emerging markets and online pharmacy platforms supports growth. Collaborations with biotech firms for therapy distribution further strengthen the segment. The trend toward decentralized healthcare and home-based treatment accelerates adoption.
Nucleic Acid-Based Therapeutics Market Regional Analysis
- North America dominated the nucleic acid-based therapeutics market with the largest revenue share of 42.5% in 2024, supported by well-established biotechnology infrastructure, significant R&D investments, high healthcare expenditure, and early adoption of advanced therapeutics, with the U.S. leading in clinical trials and commercialization of innovative nucleic acid therapies
- Healthcare providers in the region highly value the efficacy, precision, and targeted treatment potential offered by nucleic acid-based therapeutics for genetic and rare diseases, enabling personalized treatment strategies and improved patient outcomes
- This widespread adoption is further supported by favorable regulatory frameworks, strong clinical trial networks, advanced genomic research capabilities, and high healthcare expenditure, establishing North America as a key hub for both the development and commercialization of nucleic acid-based therapies
U.S. Nucleic Acid-Based Therapeutics Market Insight
The U.S. nucleic acid-based therapeutics market captured the largest revenue share of 81% in North America in 2024, driven by the rapid adoption of advanced genetic therapies and the growing emphasis on personalized medicine. Healthcare providers are increasingly prioritizing precision treatments for rare and monogenic disorders, which has accelerated clinical adoption. The presence of major biotechnology and pharmaceutical companies, combined with a robust clinical trial ecosystem, further propels market growth. In addition, strong regulatory support for orphan drugs and breakthrough therapies encourages investment and faster commercialization of novel nucleic acid therapeutics. Rising awareness among patients and physicians about the efficacy and safety of these therapies also contributes significantly to market expansion. The integration of advanced diagnostics and genomic profiling in U.S. hospitals enhances treatment accuracy and patient outcomes, strengthening market leadership.
Europe Nucleic Acid-Based Therapeutics Market Insight
The Europe nucleic acid-based therapeutics market is projected to expand at a substantial CAGR during the forecast period, driven by stringent healthcare regulations and increasing demand for precision medicine across residential and hospital-based care. Rising prevalence of genetic and rare diseases is prompting adoption of nucleic acid-based therapies. Countries such as Germany, France, and Switzerland are witnessing significant growth due to strong research infrastructure and government-backed initiatives supporting biopharmaceutical development. European healthcare systems value efficacy, safety, and advanced treatment modalities, which encourages the adoption of these therapies. Increasing collaborations between biotech firms and hospitals, along with awareness programs for patients, are enhancing market penetration. Investments in R&D and clinical trials further facilitate the commercialization of innovative therapies.
U.K. Nucleic Acid-Based Therapeutics Market Insight
The U.K. nucleic acid-based therapeutics market is expected to grow at a noteworthy CAGR, driven by increasing adoption of personalized medicine and targeted genetic treatments. Concerns regarding rare diseases and genetic disorders are motivating both healthcare providers and patients to adopt nucleic acid-based solutions. The country’s advanced healthcare infrastructure, strong regulatory support for breakthrough therapies, and robust pharmaceutical ecosystem are key growth enablers. Increasing clinical trial activity and collaboration with biotech startups further stimulate market expansion. The growing awareness among physicians about gene-targeted therapies enhances adoption. U.K.’s emphasis on innovation and healthcare modernization supports sustained growth of nucleic acid-based therapeutics.
Germany Nucleic Acid-Based Therapeutics Market Insight
The Germany nucleic acid-based therapeutics market is expected to expand at a considerable CAGR, fueled by increasing awareness of genetic disorders and the demand for innovative treatment solutions. Germany’s well-developed healthcare infrastructure and strong focus on R&D enable early adoption of nucleic acid-based therapies. Patients and healthcare providers prefer therapies that provide precise targeting and long-term efficacy, aligning with local regulatory standards. The integration of genomic research with clinical applications is also promoting market growth. Government incentives for advanced biopharmaceuticals and breakthrough therapies further facilitate commercialization. Strong collaborations between hospitals, research institutes, and biotech companies enhance accessibility and adoption.
Asia-Pacific Nucleic Acid-Based Therapeutics Market Insight
The Asia-Pacific nucleic acid-based therapeutics market is poised to grow at the fastest CAGR during the forecast period, driven by increasing prevalence of genetic disorders, expanding healthcare infrastructure, and rising adoption of precision medicine in countries such as China, Japan, and India. Government initiatives supporting digital healthcare and biotechnology R&D are boosting market penetration. The region’s growing focus on advanced therapies for rare and chronic diseases, along with increasing patient awareness, is accelerating adoption. Manufacturing capabilities and cost advantages in APAC are improving accessibility. Collaboration between global pharmaceutical companies and local healthcare providers is enhancing distribution and adoption. Expansion of clinical trials and research centers across the region further strengthens growth opportunities.
Japan Nucleic Acid-Based Therapeutics Market Insight
The Japan nucleic acid-based therapeutics market is gaining momentum due to the country’s advanced healthcare infrastructure, high focus on precision medicine, and increasing prevalence of genetic disorders. Hospitals and research institutes actively adopt nucleic acid-based therapies to improve treatment outcomes. Integration with genomic profiling and diagnostic technologies enhances therapy personalization. The aging population in Japan also drives demand for innovative treatments for chronic and rare diseases. Government support and regulatory incentives facilitate faster commercialization of advanced therapies. Collaboration with global biotech firms strengthens clinical adoption and expands market reach.
India Nucleic Acid-Based Therapeutics Market Insight
The India nucleic acid-based therapeutics market accounted for the largest revenue share in Asia-Pacific in 2024, driven by rising awareness of genetic disorders, expanding healthcare infrastructure, and increasing adoption of advanced therapies. India’s growing focus on personalized medicine, alongside supportive government initiatives and investments in biotechnology, is accelerating market growth. The availability of affordable therapies and local manufacturing capabilities improve accessibility. Hospitals and specialty clinics are increasingly offering nucleic acid-based treatments for rare and chronic diseases. Partnerships with global pharmaceutical companies are enhancing technology transfer and commercialization. Expansion of clinical research and patient education programs further supports market adoption.
Nucleic Acid-Based Therapeutics Market Share
The nucleic acid-based therapeutics industry is primarily led by well-established companies, including:
- Ionis Pharmaceuticals (U.S.)
- Vertex Pharmaceuticals Incorporated (U.S.)
- CRISPR Therapeutics (Switzerland)
- BioNTech SE (Germany)
- CureVac SE (Germany)
- Biogen Inc. (U.S.)
- TransCode Therapeutics, Inc. (U.S.)
- Regeneron Pharmaceuticals, Inc. (U.S.)
- Moderna, Inc. (U.S.)
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Intellia Therapeutics, Inc. (U.S.)
- Editas Medicine, Inc. (U.S.)
- Beam Therapeutics Inc. (U.S.)
- Sangamo Therapeutics, Inc. (U.S.)
- Novartis AG (Switzerland)
- Arcturus Therapeutics Holdings Inc. (U.S.)
- Regulus Therapeutics Inc. (U.S.)
- Sanofi (France)
- Gilead Sciences, Inc. (U.S.)
- Pfizer Inc. (U.S.)
What are the Recent Developments in Global Nucleic Acid-Based Therapeutics Market?
- In August 2025, Ionis Pharmaceuticals announced the U.S. launch of Dawnzera (donidalorsen), the first RNA-targeted therapy approved as a prophylactic treatment for hereditary angioedema (HAE). This approval marks a significant advancement in RNA-based therapeutics for rare diseases
- In August 2025, Arnatar Therapeutics debuted with ART4, an antisense oligonucleotide designed to upregulate protein expression for treating Alagille syndrome. The FDA granted both Orphan Drug and Rare Pediatric Disease designations to ART4, highlighting its potential for addressing unmet medical needs
- In May 2025, Biogen and City Therapeutics entered a strategic research collaboration to develop novel RNA interference (RNAi)-based therapies targeting central nervous system diseases. The partnership combines City Therapeutics' RNAi engineering technologies with Biogen's drug development expertise
- In September 2024, ME Therapeutics commenced efficacy testing of the first mRNA formulations developed through its collaboration with NanoVation Therapeutics. The partnership focuses on advancing RNA-based cancer immunotherapies
- In January 2024, Therapeutics and Debiopharm announced a co-research agreement to develop targeted nucleic acid therapeutics for cancer. The collaboration aims to combine TransCode's TTX delivery platform with Debiopharm's expertise in targeted drug delivery
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.