Global Ocular Loteprednol Market
Market Size in USD Million
CAGR :
% 
 USD
99.06 Million
USD
168.93 Million
2024
2032
USD
99.06 Million
USD
168.93 Million
2024
2032
| 2025 –2032 | |
| USD 99.06 Million | |
| USD 168.93 Million | |
|
|
|
|
Ocular Loteprednol Market Size
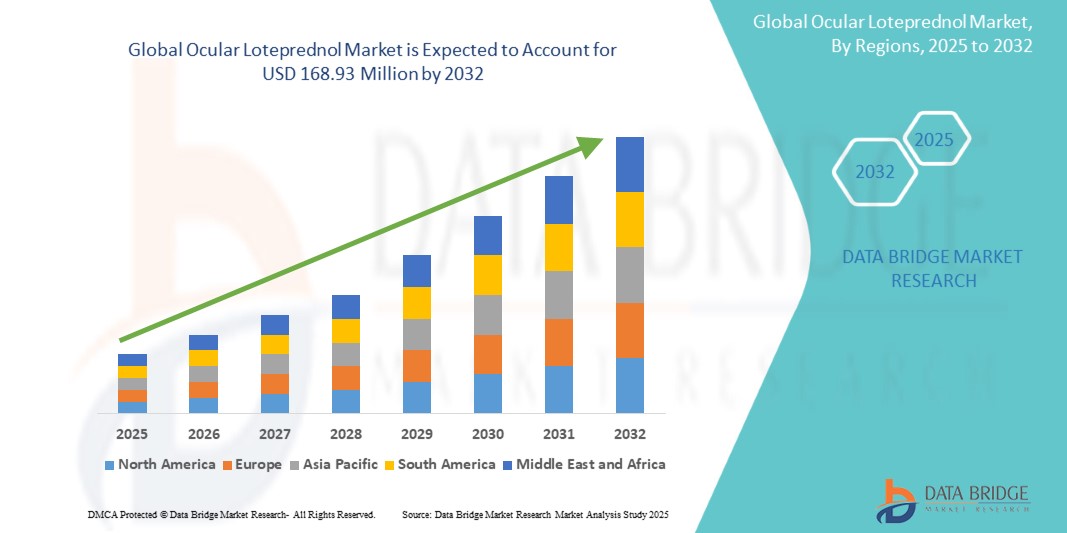
- The global ocular loteprednol market size was valued at USD 99.06 million in 2024 and is expected to reach USD 168.93 million by 2032, at a CAGR of 6.90% during the forecast period
- The market growth is largely driven by the increasing prevalence of ocular inflammatory conditions such as allergic conjunctivitis, uveitis, and post-operative inflammation, along with rising awareness of advanced ophthalmic therapies
- Furthermore, the drug’s favorable safety profile, reduced risk of steroid-induced side effects, and availability in multiple patient-friendly formulations are strengthening its adoption across hospitals, clinics, and self-care settings. These converging factors are accelerating the uptake of ocular loteprednol treatments, thereby significantly boosting the industry’s growth
Ocular Loteprednol Market Analysis
- Ocular loteprednol, a corticosteroid used in ophthalmology, is increasingly vital in the treatment of inflammatory eye disorders such as allergic conjunctivitis, uveitis, dry eye disease, keratitis, and post-operative inflammation, due to its strong anti-inflammatory efficacy combined with a reduced risk of steroid-related side effects such as intraocular pressure elevation
- The escalating demand for ocular loteprednol is primarily fueled by the rising prevalence of ocular diseases, an aging global population, increasing ophthalmic surgical procedures, and the growing preference for safer, targeted corticosteroid therapies in eye care
- North America dominated the ocular loteprednol market with the largest revenue share of 40.3% in 2024, supported by advanced healthcare infrastructure, high awareness among ophthalmologists, and a strong presence of leading pharmaceutical companies, with the U.S. showing substantial growth driven by product approvals, patient adoption, and expanding insurance coverage
- Asia-Pacific is expected to be the fastest growing region in the ocular loteprednol market during the forecast period, attributed to rising cases of ocular allergies, expanding access to eye care, increasing healthcare expenditure, and growing adoption of branded and generic formulations
- Loteprednol Eye Drops segment dominated the ocular loteprednol market with a market share of 46.7% in 2024, driven by their ease of administration, rapid therapeutic action, and widespread use in both acute and chronic ocular inflammatory conditions
Report Scope and Ocular Loteprednol Market Segmentation
|
Attributes |
Ocular Loteprednol Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Ocular Loteprednol Market Trends
Rising Adoption of Safer Corticosteroids in Ophthalmology
- A significant and accelerating trend in the global ocular loteprednol market is the growing preference for ester-based corticosteroids, such as loteprednol, over traditional ketone-based steroids such as prednisolone and dexamethasone. This shift is driven by loteprednol’s strong anti-inflammatory efficacy with a reduced risk of side effects, particularly steroid-induced intraocular pressure elevation and cataract formation
- For instance, branded formulations such as Lotemax SM and Alrex are widely prescribed in the U.S. for allergic conjunctivitis and post-operative inflammation, offering ophthalmologists a safer long-term alternative for chronic ocular inflammatory conditions
- Pharmaceutical companies are increasingly innovating with advanced drug delivery platforms, such as gels, ointments, and nano-suspensions, to improve bioavailability and patient compliance. These formulations ensure prolonged ocular surface retention, reduced dosing frequency, and better therapeutic outcomes
- Moreover, loteprednol is being incorporated into combination therapies with lubricants or antihistamines to broaden its therapeutic scope in conditions such as dry eye disease and seasonal allergies. Such integrations are enhancing patient convenience while expanding commercial potential
- Regulatory approvals for new formulations and expanding generic availability in emerging markets are accelerating its adoption worldwide, strengthening the drug’s role in first-line ocular inflammation management
- This trend toward safer, more patient-friendly corticosteroid options is reshaping treatment strategies in ophthalmology, positioning ocular loteprednol as a preferred choice among both specialists and patients globally
Ocular Loteprednol Market Dynamics
Driver
Growing Prevalence of Ocular Inflammatory Disorders and Surgical Interventions
- The rising global incidence of allergic conjunctivitis, uveitis, keratitis, and dry eye disease, coupled with the increasing number of cataract and refractive surgeries, is significantly driving demand for ocular loteprednol
- For instance, in 2024, cataract surgeries surpassed 30 million annually worldwide, with loteprednol frequently prescribed post-operatively due to its favorable safety profile compared to older corticosteroids. Such surgical growth directly boosts the demand for ocular steroids
- Patients and ophthalmologists asuch as are increasingly prioritizing drugs with lower side effect risks, making loteprednol an attractive option for long-term management of chronic ocular conditions
- Furthermore, rising healthcare access in Asia-Pacific and Latin America, along with expanding insurance coverage in developed countries, is increasing prescription volumes and market penetration
- The availability of multiple dosage forms eye drops, ointments, gels, and suspensions further enhances adoption across diverse patient needs in both hospital and homecare settings.
Restraint/Challenge
High Cost of Branded Formulations and Limited Awareness in Developing Regions
- Despite its benefits, the high cost of branded formulations such as Lotemax poses a barrier to adoption in price-sensitive markets, particularly when cheaper alternatives such as prednisolone or dexamethasone are available
- For instance, loteprednol products can cost 3–4 times more than generic corticosteroids, creating affordability issues in emerging economies where out-of-pocket expenditure remains high
- Limited awareness among general practitioners and optometrists in developing countries also restricts wider adoption, as many continue to prescribe older steroids despite their higher risk profiles
- Moreover, the introduction of generics, while expanding accessibility, also intensifies price competition, putting pressure on margins for branded drug manufacturers
- Overcoming these challenges will require a focus on education programs for prescribers, expansion of cost-effective generic formulations, and strategic partnerships to enhance distribution in underpenetrated regions
Ocular Loteprednol Market Scope
The market is segmented on the basis of formulation type, application, distribution channel, and end user.
- By Formulation Type
On the basis of formulation type, the ocular loteprednol market is segmented into loteprednol eye drops, ointments, gels, and suspensions. The eye drops segment dominated the market with the largest revenue share of 46.7%, driven by its first-line positioning across allergic conjunctivitis, dry eye flares, and post-operative inflammation. Eye drops offer simple, familiar administration and rapid symptom relief, supporting high patient adherence in both acute and chronic care. Broad availability of branded and generic SKUs across regions strengthens formulary access and affordability. Clinicians favor drops for dose titration flexibility and minimal chair time compared with in-office procedures.
The suspensions segment is anticipated to witness the fastest growth rate during the forecast period, propelled by advances in nano- and micro-suspension technologies that enhance corneal penetration and residence time. Reduced dosing frequency improves persistence in chronic conditions such as uveitis and keratitis. Newer rheology profiles minimize blur and improve comfort versus older suspensions, aiding switch conversions. Pipeline innovation and regional approvals are expanding prescriber confidence and geographic reach. As payers and providers seek outcomes with fewer instillations, optimized suspensions gain traction in step-up therapy.
- By Application
On the basis of application, the ocular loteprednol market is segmented into allergic conjunctivitis, dry eye disease, uveitis, post-operative inflammation, keratitis, and others. Allergic conjunctivitis dominated the market owing to its high and rising global prevalence across urban populations and longer allergy seasons. Loteprednol’s ester-based profile provides strong anti-inflammatory relief with a lower risk of IOP spikes, enabling recurrent and seasonal use. Rapid itch and redness control positions it as a rescue therapy alongside antihistamine/mast-cell stabilizers. The large primary-care and optometry base broadens prescribing beyond tertiary ophthalmology. OTC co-usage with lubricants further increases refill velocity and chronicity. Environmental triggers and screen-time–related ocular surface stress continue to expand the treatable pool, reinforcing leadership.
Post-operative inflammation is expected to be the fastest growing segment, supported by rising cataract and refractive procedure volumes worldwide. Surgeons increasingly prefer loteprednol for elderly and steroid-responsive patients due to its favorable safety profile. Standardized peri-operative protocols and bundled discharge kits drive predictable utilization. Shorter taper schedules and good tolerability improve adherence in the critical healing window. Expansion of day-care surgery centers in emerging markets widens access and script counts. As premium IOL and refractive segments grow, demand for reliable, comfort-oriented steroids accelerates usage post-op.
- By Distribution Channel
On the basis of distribution channel, the ocular loteprednol market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies held the dominant share, as a large proportion of loteprednol prescriptions originate peri-operatively and from acute ophthalmic consults. Embedded care pathways streamline prescribing and dispensing before patient discharge. Hospital formularies and purchasing groups secure steady throughput and supply continuity. Close surgeon–pharmacist coordination ensures adherence to post-op regimens and timely refills. Diagnostic capability within hospitals captures severe inflammatory cases that necessitate steroid therapy.
Online pharmacies are projected to record the fastest growth, driven by tele-ophthalmology adoption and e-prescription penetration. Chronic allergic and dry eye patients value home delivery, auto-refill options, and transparent pricing. Digital platforms expand access to both branded and generic loteprednol in under-served geographies. App-based adherence reminders and therapy education improve persistence and lifetime value per patient. Partnerships with e-clinics and virtual consult services funnel qualified prescriptions directly to e-pharmacies. As regulations mature and verification systems strengthen, consumer trust accelerates the channel shift.
- By End User
On the basis of end user, the ocular loteprednol market is segmented into hospitals, ophthalmic clinics, and homecare/self-administration. Hospitals dominated the market, reflecting their central role in surgical care and acute inflammation management. Integrated diagnostics and same-day treatment pathways convert a high share of encounters into steroid prescriptions. Access to subspecialists (cornea, uveitis) concentrates complex cases requiring controlled steroid tapers. Institutional protocols and stocked inventories ensure immediate therapy initiation, reducing complications. Reimbursement alignment in developed markets supports formulary placement and steady utilization. Post-discharge coordination with hospital pharmacies further amplifies script capture.
Homecare/self-administration is expected to grow the fastest, underpinned by the shift toward patient-managed chronic ocular conditions. User-friendly drop and suspension formats enable safe, convenient dosing without in-clinic procedures. Digital adherence tools, pharmacist counseling, and clearer label instructions improve outcomes at home. Broader retail and online access lowers friction for refills and therapy continuity. As patients and caregivers gain confidence with tapering schedules, real-world persistence improves. Demographic aging and rising allergy/dry-eye prevalence expand the home-managed population, accelerating growth in this end-user segment.
Ocular Loteprednol Market Regional Analysis
- North America dominated the ocular loteprednol market with the largest revenue share of 40.3% in 2024, supported by advanced healthcare infrastructure, high awareness among ophthalmologists, and a strong presence of leading pharmaceutical companies
- Consumers and patients in North America particularly value the efficacy, tolerability, and ease of self-administration offered by loteprednol formulations such as eye drops and suspensions. The market also benefits from the increasing adoption of tele-ophthalmology services, which facilitate e-prescriptions and broader drug access through both hospital and online pharmacies
- With the region’s aging population and a high rate of cataract and refractive surgeries, the demand for peri-operative and chronic inflammation management solutions is set to remain robust. Strong collaboration between healthcare providers, payers, and pharma companies ensures continuous uptake, reinforcing the region’s dominant share
The U.S. Ocular Loteprednol Market Insight
The U.S. ocular loteprednol market captured the largest revenue share of 79% in 2024 within North America, driven by the high prevalence of allergic conjunctivitis, dry eye disease, and post-operative ocular inflammation. The country’s strong clinical adoption of safer corticosteroid therapies, combined with high healthcare expenditure, accelerates demand. Physicians increasingly prefer loteprednol due to its lower risk of intraocular pressure elevation compared to traditional steroids. The presence of leading pharmaceutical companies, well-established reimbursement systems, and expanding tele-ophthalmology adoption further strengthen its market dominance.
Europe Ocular Loteprednol Market Insight
The Europe ocular loteprednol market is projected to expand at a substantial CAGR during the forecast period, supported by growing awareness of ocular surface diseases and post-surgical inflammation management. Rising ophthalmic surgical procedures such as cataract and refractive surgeries are significantly contributing to demand for corticosteroid-based eye therapies. European consumers benefit from widespread availability through hospital and retail pharmacies, alongside regulatory emphasis on safe and effective ophthalmic drugs. The region is also witnessing increased clinical trials and generic drug approvals, further supporting long-term growth in both developed and emerging European markets.
U.K. Ocular Loteprednol Market Insight
The U.K. ocular loteprednol market is anticipated to grow at a noteworthy CAGR, fueled by rising cases of dry eye disease and allergic conjunctivitis. The growing preference for advanced corticosteroid formulations with minimal side effects is enhancing adoption across ophthalmic clinics and hospitals. In addition, strong public healthcare infrastructure, combined with private sector distribution through retail and online pharmacies, ensures broad patient access. Increasing surgical interventions and aging demographics are further boosting demand for anti-inflammatory eye therapies.
Germany Ocular Loteprednol Market Insight
The Germany ocular loteprednol market is expected to expand at a considerable CAGR, driven by a well-established healthcare infrastructure and emphasis on innovation in ophthalmology. High prevalence of chronic eye diseases and increased surgical procedures are fostering demand for safe corticosteroid therapies. Germany’s focus on quality, eco-conscious pharmaceutical solutions, and its role as a hub for clinical research and drug development make it a key growth market. In addition, the integration of digital healthcare solutions is improving prescription access and patient compliance, supporting loteprednol adoption.
Asia-Pacific Ocular Loteprednol Market Insight
The Asia-Pacific ocular loteprednol market is poised to grow at the fastest CAGR of 23.5% from 2025 to 2032, fueled by increasing urbanization, rising disposable incomes, and higher awareness of eye health. Countries such as China, Japan, and India are seeing rapid growth in ophthalmic surgeries and an expanding patient pool for chronic eye conditions. Government initiatives to improve healthcare access and rising investments by pharmaceutical companies are accelerating drug availability. Moreover, the region’s growing middle-class population and demand for affordable ophthalmic care are propelling adoption of loteprednol therapies.
Japan Ocular Loteprednol Market Insight
The Japan ocular loteprednol market is gaining momentum due to the country’s advanced healthcare system and high patient awareness of ophthalmic treatments. Rapid urbanization and increasing cases of ocular surface diseases are driving demand for corticosteroid-based therapies. Japan’s strong focus on technological innovation ensures quick integration of new formulations, including preservative-free and patient-friendly dosage forms. In addition, its aging population is expected to further boost prescriptions, particularly for post-operative inflammation and chronic ocular conditions.
India Ocular Loteprednol Market Insight
The India ocular loteprednol market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the country’s expanding middle-class population, rising eye disease prevalence, and affordability of generic loteprednol formulations. Rapid urbanization, high rates of cataract surgeries, and government-led initiatives for eye care access are fueling growth. Domestic pharmaceutical manufacturers play a critical role in ensuring competitive pricing and wide availability. With growing adoption of digital health platforms and online pharmacies, India continues to emerge as a key hub for ocular loteprednol demand.
Ocular Loteprednol Market Share
The Ocular Loteprednol industry is primarily led by well-established companies, including:
- Bausch + Lomb (Canada)
- Alcon (Switzerland)
- Lupin Limited (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Amneal Pharmaceuticals, Inc. (U.S.)
- Padagis US LLC (U.S.)
- Sentiss Pharma Pvt. Ltd. (India)
- Armas Pharmaceuticals, Inc. (U.S.)
- Kala Pharmaceuticals, Inc. (U.S.)
- Bausch Health Companies Inc. (Canada)
- Cipla Limited (India)
- Ajanta Pharma Ltd. (India)
- Alembic Pharmaceuticals Ltd. (India)
- Zydus Lifesciences Ltd. (India)
- Dr. Reddy’s Laboratories Ltd. (India)
- Glenmark Pharmaceuticals Ltd. (India)
- Indoco Remedies Ltd. (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Apotex Inc. (Canada)
- Intas Pharmaceuticals Ltd. (India)
What are the Recent Developments in Global Ocular Loteprednol Market?
- In July 2025, Lupin launched its generic version of loteprednol etabonate ophthalmic suspension (0.5%) in the U.S., bioequivalent to Bausch & Lomb’s Lotemax Suspension, opening a cost-effective alternative for treating steroid-responsive ocular inflammation
- In January 2025, Lupin announced receipt of tentative FDA approval for its Loteprednol Etabonate Ophthalmic Gel, 0.38%, pending exclusivity as the first-to-file ANDA. This gel is bioequivalent to Lotemax SM Gel and targets post-operative inflammation, with a significant US annual sales reference
- In December 2023, Lupin received U.S. FDA approval for its generic Loteprednol Etabonate Ophthalmic Suspension, 0.2%, a counterpart to Alrex® (0.2%), aimed at treating seasonal allergic conjunctivitis. This approval expands patient access to a cost-effective steroid alternative for ocular allergies
- In May 2022, Alcon announced its acquisition of Eysuvis and Inveltys from Kala Pharmaceuticals, broadening its ophthalmic portfolio with loteprednol-based therapies for dry eye disease and postoperative inflammation. The acquisition included Eysuvis (loteprednol etabonate suspension 0.25%) and Inveltys (1%) and was part of a strategic move to expand treatment options in eye care
- In May 2022, Kala Pharmaceuticals entered into a definitive asset-sale agreement to transfer its commercial portfolio—Eysuvis (loteprednol etabonate suspension 0.25%) and Inveltys® (loteprednol etabonate suspension 1%)—to Alcon Inc., enabling broader global reach of these loteprednol-based therapies under Alcon's established ophthalmic platform and strengthening both companies' strategic focus
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.