Global Osteoporosis Drug Market
Market Size in USD Million
CAGR :
% 
 USD
15,576.30 Million
USD
20,332.57 Million
2022
2030
USD
15,576.30 Million
USD
20,332.57 Million
2022
2030
| 2023 –2030 | |
| USD 15,576.30 Million | |
| USD 20,332.57 Million | |
|
|
|
|
Osteoporosis Drug Market Size
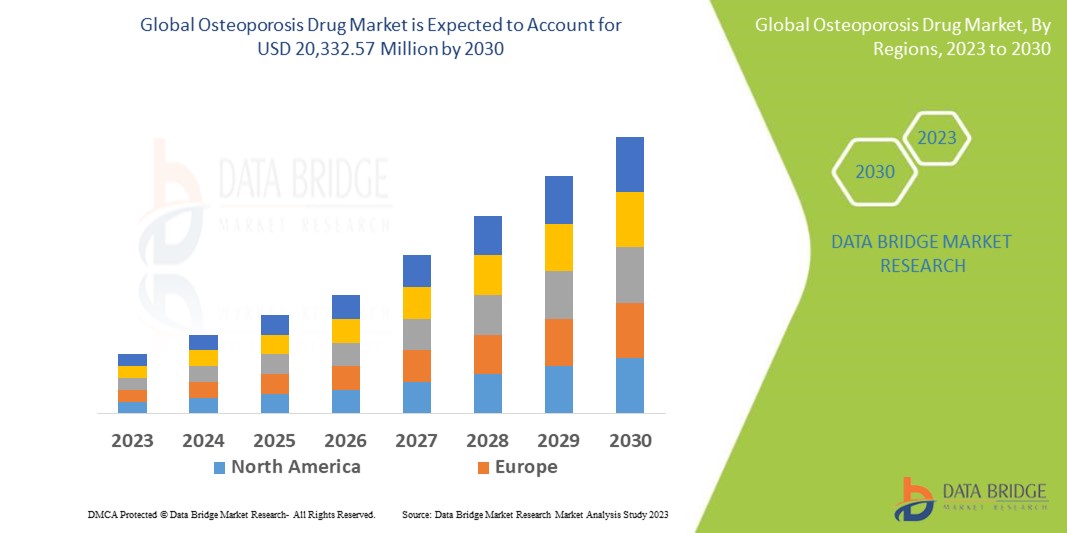
The global osteoporosis drug market is experiencing steady growth driven by factors such as the increasing aging population, rising prevalence of osteoporosis, and growing awareness about bone health. Data Bridge Market Research analyses that the osteoporosis drug market which was USD 15,576.30 million in 2022, would rocket up to USD 20,332.57 million by 2030, and is expected to undergo a CAGR of 4.68% during the forecast period. This indicates that the market value. “Medication” dominates the treatment type segment of the osteoporosis drug market owing to the growing demand for medication treatment due to rise in prevalence of osteoporosis globally. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Osteoporosis Drug Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021(Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
By Type (Primary Osteoporosis and Secondary Osteoporosis), Therapy Type (Hormone Replacement Therapy and Bisphosphonate Therapy), Treatment Type (Medication and Surgery), Mechanism of Action Type (Bisphosphonates, Selective Estrogen Receptor Modulators and Bone Resorption Inhibitors), Route of Administration Type (Oral, Intravenous, Subcutaneous and Others), End-Users (Hospitals, Homecare, Specialty Clinics and Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
GlaxoSmithKline plc. (U.K.), F. Hoffmann-La Roche Ltd (Switzerland), Novartis AG (Switzerland), Eli Lilly And Company. (U.S.), Astrazeneca (U.K.), Pfizer Inc. (U.S.), Takeda Pharmaceutical Company Limited. (Japan), Bristol-Myers Squibb Company (U.S.), Sanofi (France), Johnson & Johnson Services, Inc.(U.S.), Bayer AG (Germany), AbbVie Inc. (U.S.), Allergan (Ireland), Merck & Co., Inc. (U.S.), Amgen Inc. (U.S.), Sun Pharmaceutical Industries Ltd. (India), Teva Pharmaceutical Industries Ltd. (Israel), Novo Nordisk A/S (Denmark), DAIICHI SANKYO COMPANY (Japan), LIMITED. and Cipla Inc. (India) |
|
Market Opportunities |
|
Market Definition
Osteoporosis is a condition of bone weakening or bones becomes fragile and likely to break. In other words, osteoporosis is a condition wherein the bone density decreases. This is specially witnessed in old age people. Osteoporosis brings along back pain, stooped posture, bone fracture and tooth loss.
Osteoporosis Drug Market Dynamics
Drivers
- Increasing Prevalence of Osteoporosis
The prevalence of osteoporosis is increasing worldwide mainly due to changing lifestyles, poor diet and sedentary lifestyle. Factors that contribute to osteoporosis, such as reduced physical activity, insufficient intake of calcium and vitamin D and hormonal imbalances. The increasing number of people suffering from osteoporosis is creating a significant demand for osteoporosis drugs
- Increased Awareness and Diagnosis
Awareness of osteoporosis and its consequences has increased significantly among both health professionals and the general public. Initiatives by health organizations, patient advocacy groups, and pharmaceutical companies have played an important role in educating people about osteoporosis prevention, early detection, and available treatment options. As a result, more and more people are diagnosed with osteoporosis, increasing the demand for osteoporosis drugs
- Technological Advances in Drug Development
Technological advances in drug development have made it possible to discover and develop more effective and targeted treatments for osteoporosis. The use of advanced imaging techniques, molecular biology and genomics has provided insight into the pathophysiology of osteoporosis and led to the identification of new drug targets. The development of new drug delivery systems and formulation techniques will also improve the efficacy and patient compliance of osteoporosis medications
- Growing Investment for Healthcare Facilities
Surging focus towards improving the condition of healthcare facilities and improving the overall healthcare infrastructure another important factor fostering the growth of the market. Rising number of partnerships and strategic collaborations between the public and private players pertaining to funding and application of new and improved technology is further creating lucrative market opportunities
Opportunities
- Targeted Therapies and Personalized Medicine
The development of targeted therapies and personalized treatment approaches offers the opportunity to improve the effectiveness and safety of osteoporosis treatment. Advances in genomics, biomarker identification and precision medicine enable specific patient subgroups that may benefit from tailored treatment. By developing innovative treatment methods that meet the individual characteristics and needs of the patient, treatment results can be improved and products can be differentiated in the market
- Research and Development for New Drug Candidates
Ongoing research and development in the field of osteoporosis offers opportunities for the discovery and development of new drug candidates. Exploring new mechanisms of action, identifying innovative drug targets and exploiting advances in drug delivery systems can lead to the development of innovative therapies with greater efficacy and fewer side effects. Companies that invest in RandD to expand their product portfolio can take advantage of these opportunities and gain a competitive advantage in the market
Restraints
- Patent Expiration and Generic Competition
Many osteoporosis drugs have had patent expirations, allowing generic versions to enter the market. Generic drug competition often leads to lower prices, which reduces the revenue potential of originator drug manufacturers. This may affect profitability and hinder the growth of the osteoporosis drug market
- Side Effects and Safety Concerns
Osteoporosis medications, such as all medications, can have potential side effects and safety concerns. Some medications have been associated with rare but serious side effects, such as atypical fractures and osteonecrosis of the jaw. These safety concerns may lead to regulatory actions, warnings, and restrictions affecting the prescribing and marketing of certain osteoporosis drugs
Challenges
- High Development Costs and Long Development Times
Developing and marketing a new osteoporosis drug requires significant research and development costs, including preclinical studies, clinical trials and regulatory notifications. The process can be time-consuming and often takes years from initial discovery to commercialization. In addition, the high failure rate of clinical trials further increases costs and lengthens development schedules. These challenges increase the financial burden on pharmaceutical companies and create uncertainty about the return on invested capital
- Reimbursement and Pricing Pressures
Osteoporosis drugs, especially the newer biologics, can be expensive. Pricing pressures from health systems, payers and insurance companies can limit the availability and affordability of these drugs. Reimbursement policies, formulary restrictions, and cost containment measures can affect market access, patient affordability, and overall market demand. Pharmaceutical companies must overcome these challenges to secure favourable reimbursement and pricing agreements while demonstrating the value and cost-effectiveness of their products
This osteoporosis drug market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the Osteoporosis Drug market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth
Recent Developments
- In August 2021, Enzene Biosciences Ltd received Marketing Authorization (MA) from the Drug Controller General of India (DCGI) for its biosimilar drug, denosumab, indicated for the treatment of osteoporosis in adults
- In January 2021, Theramex, a London-headquartered pharmaceutical company, launched the osteoporosis medicine Livogiva in Europe
Global Osteoporosis Drug Market Scope
The osteoporosis drug market is segmented on the basis of type, therapy type, treatment type, mechanism of action, route of administration and end users. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Primary osteoporosis
- Postmenopausal osteoporosis
- Senile osteoporosis
- Idiopathic osteoporosis
- Seocndary osteoporosis
Therapy type
- Hormone Replacement Therapy
- Testosterone Replacement Therapy
- Estrogen Therapy
- Bisphosphonate Therapy
Treatment type
- Medication
- Calcium
- Vitamin D Supplements
- Antacids
- Surgery
- Vertebroplasty
- Kyphoplasty
- Others
Mechanism of Action
- Bisphosphonates
- Alendronate
- Ibandronate
- Risedronate
- Zoledronic
- Selective Estrogen Receptor Modulators
- Raloxifene
- Bone Resorption Inhibitors
- Denosumab
Route of Administration
- Oral
- Intravenous
- Subcutaneous
- Others
End-User
- Hospitals
- Homecare
- Specialty clinics
- Others
Osteoporosis Drug Market Regional Analysis/Insights
The osteoporosis drug market is analysed and market size insights and trends are provided by country, type, therapy type, treatment type, mechanism of action, route of administration and end-users as referenced above.
The countries covered in the osteoporosis drug market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
North America dominates the osteoporosis drug market owing to the easy availability of osteoporosis drugs and rising prevalence of osteoporosis.
Asia-Pacific is projected to score highest growth rate for the forecast period owing to rising expenditure to develop healthcare infrastructure coupled with growth and expansion of life sciences industry.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and up-stream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The Osteoporosis drug market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for Osteoporosis drug market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the Osteoporosis Drug market. The data is available for historic period from 2021 to 2030.
Competitive Landscape and Osteoporosis Drug Market Share Analysis
The osteoporosis drug market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the company’s focus related to osteoporosis drug market.
Some of the major players operating in the osteoporosis drug market are
- GlaxoSmithKline plc. (U.K.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Novartis AG (Switzerland)
- Eli Lilly And Company. (U.S.)
- Astrazeneca (U.K.)
- Pfizer Inc. (U.S.)
- Takeda Pharmaceutical Company Limited. (Japan)
- Bristol-Myers Squibb Company (U.S.)
- Sanofi (France)
- Johnson & Johnson Services, Inc.(U.S.)
- Bayer AG (Germany)
- AbbVie Inc. (U.S.)
- Allergan (Ireland)
- Merck & Co., Inc. (U.S.)
- Amgen Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Novo Nordisk A/S (Denmark)
- DAIICHI SANKYO COMPANY (Japan), LIMITED.
- Cipla Inc. (India)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL OSTEOPOROSIS DRUG MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL OSTEOPOROSIS DRUG MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 MARKET GUIDE
2.2.4 COMPANY POSITIONING GRID
2.2.5 COMPANY MARKET SHARE ANALYSIS
2.2.6 MULTIVARIATE MODELLING
2.2.7 TOP TO BOTTOM ANALYSIS
2.2.8 STANDARDS OF MEASUREMENT
2.2.9 VENDOR SHARE ANALYSIS
2.2.10 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.11 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL OSTEOPOROSIS DRUG MARKET : RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10 PIPELINE ANALYSIS
10.1 CLINICAL TRIALS AND PHASE ANALYSIS
10.2 DRUG THERAPY PIPELINE
10.3 PHASE III CANDIDATES
10.4 PHASE II CANDIDATES
10.5 PHASE I CANDIDATES
10.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 COLOMBIA CLINICAL TRIAL MARKET FOR XX
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
11 MARKETED DRUG ANALYSIS
11.1 DRUG
11.1.1 BRAND NAME
11.1.2 GENERICS NAME
11.2 THERAPEUTIC INDIACTION
11.3 PHARACOLOGICAL CLASS OD THE DRUG
11.4 DRUG PRIMARY INDICATION
11.5 MARKET STATUS
11.6 MEDICATION TYPE
11.7 DRUG DOSAGES FORM
11.8 DOSAGES AVAILABILITY
11.9 PACKAGING TYPE
11.1 DRUG ROUTE OF ADMINISTRATION
11.11 DOSING FREQUENCY
11.12 DRUG INSIGHT
11.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
11.13.1 FORECAST MARKET OUTLOOK
11.13.2 CROSS COMPETITION
11.13.3 THERAPEUTIC PORTFOLIO
11.13.4 CURRENT DEVELOPMENT SCENARIO
12 MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13 R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14 MARKET OVERVIEW
14.1 DRIVERS
14.2 RESTRAINTS
14.3 OPPORTUNITIES
14.4 CHALLENGES
15 GLOBAL OSTEOPOROSIS DRUGS MARKET, BY TYPE
15.1 OVERVIEW
15.2 PRIMARY OSTEOPOROSIS
15.3 SECONDARY OSTEOPOROSIS
16 GLOBAL OSTEOPOROSIS DRUGS MARKET, BY THERAPY TYPE
16.1 OVERVIEW
16.2 BISPHOSPHONATES
16.2.1 ALENDRONATE
16.2.2 IBANDRONATE
16.2.3 RISEDRONATE
16.2.4 ZOLEDRONIC ACID
16.2.5 OTHERS
16.3 HORMONE THERAPY
16.3.1 PARATHYROID HORMONE
16.3.1.1. ABALOPARATIDE
16.3.1.2. TERIPARATIDE
16.3.1.3. OTHERS
16.3.2 ESTROGEN
16.3.2.1. CONJUGATED ESTROGENS
16.3.2.2. ESTERIFIED ESTROGENS
16.3.2.3. ESTROPIPATE
16.3.2.4. SELECTIVE ESTROGEN RECEPTOR MODULATORS
16.3.3 CALCITONIN
16.3.4 OTHERS
16.4 OTHERS
17 GLOBAL OSTEOPOROSIS DRUGS MARKET, BY DRUG TYPE
17.1 OVERVIEW
17.2 BRANDED
17.2.1 FOSAMAX
17.2.2 FORTEO
17.2.3 ACTONEL
17.2.4 RECLAST
17.2.5 ACLASTA
17.2.6 EVENITY
17.2.7 PROLIA
17.2.8 BINOSTO
17.2.9 OTHERS
17.3 BIOSIMILARS
17.3.1 DENOSUMAB
17.3.2 TERROSA
17.3.3 OTHERS
17.4 GENERICS
18 GLOBAL OSTEOPOROSIS DRUGS MARKET, BY MECHANISM OF ACTION
18.1 OVERVIEW
18.2 BISPHOSPHONATES
18.3 SELECTIVE ESTROGEN RECEPTOR MODULATORS
18.4 BONE RESORPTION INHIBITORS
18.5 OTHERS
19 GLOBAL OSTEOPOROSIS DRUGS MARKET, BY ROUTE OF ADMINISTRATION
19.1 OVERVIEW
19.2 ORAL
19.2.1 TABLETS
19.2.2 CAPSULES
19.2.3 OTHERS
19.3 INJECTABLE
19.4 SUBCUTANEOUS
19.5 OTHERS
20 GLOBAL OSTEOPOROSIS DRUGS MARKET, BY GENDER
20.1 OVERVIEW
20.2 MALE
20.2.1 PEDIATRIC
20.2.2 ADULTS
20.3 FEMALE
20.3.1 PEDIATRIC
20.3.2 ADULTS
21 GLOBAL OSTEOPOROSIS DRUGS MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS
21.3 HOMECARE
21.4 SPECIALTY CLINICS
21.5 OTHERS
22 GLOBAL OSTEOPOROSIS DRUGS MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDER
22.3 RETAILS SALES
22.3.1 PHARMACY STORES
22.3.2 ONLINE RETAIL CHANNELS
22.3.3 OTHERS
22.4 OTHERS
23 GLOBAL OSTEOPOROSIS DRUG MARKET, SWOT AND DBMR ANALYSIS
24 GLOBAL OSTEOPOROSIS DRUG MARKET, COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: SOUTH AMERICA
24.5 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
24.6 MERGERS & ACQUISITIONS
24.7 NEW PRODUCT DEVELOPMENT & APPROVALS
24.8 EXPANSIONS
24.9 REGULATORY CHANGES
24.1 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 GLOBAL OSTEOPOROSIS DRUG MARKET, BY REGION, 2022-2031, (USD MILLION)
GLOBAL OSTEOPOROSIS DRUG MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
25.1 OVERVIEW
25.2 NORTH AMERICA
25.2.1 U.S.
25.2.2 CANADA
25.2.3 MEXICO
25.3 EUROPE
25.3.1 GERMANY
25.3.2 U.K.
25.3.3 ITALY
25.3.4 FRANCE
25.3.5 SPAIN
25.3.6 SWITZERLAND
25.3.7 RUSSIA
25.3.8 TURKEY
25.3.9 BELGIUM
25.3.10 NETHERLANDS
25.3.11 REST OF EUROPE
25.4 ASIA-PACIFIC
25.4.1 JAPAN
25.4.2 CHINA
25.4.3 SOUTH KOREA
25.4.4 INDIA
25.4.5 AUSTRALIA & NEW ZEALAND
25.4.6 SINGAPORE
25.4.7 THAILAND
25.4.8 INDONESIA
25.4.9 MALAYSIA
25.4.10 PHILIPPINES
25.4.11 REST OF ASIA-PACIFIC
25.5 SOUTH AMERICA
25.5.1 BRAZIL
25.5.2 ARGENTINA
25.5.3 REST OF SOUTH AMERICA
25.6 MIDDLE EAST AND AFRICA
25.6.1 SOUTH AFRICA
25.6.2 EGYPT
25.6.3 SAUDI ARABIA
25.6.4 UNITED ARAB EMIRATES
25.6.5 ISRAEL
25.6.6 REST OF MIDDLE EAST AND AFRICA
26 GLOBAL OSTEOPOROSIS DRUG MARKET, COMPANY PROFILE
26.1 AMGEN INC
26.1.1 COMPANY OVERVIEW
26.1.2 REVENUE ANALYSIS
26.1.3 GEOGRAPHIC PRESENCE
26.1.4 PRODUCT PORTFOLIO
26.1.5 RECENT DEVELOPMENTS
26.2 NOVARTIS AG
26.2.1 COMPANY OVERVIEW
26.2.2 REVENUE ANALYSIS
26.2.3 GEOGRAPHIC PRESENCE
26.2.4 PRODUCT PORTFOLIO
26.2.5 RECENT DEVELOPMENTS
26.3 AUROBINDO PHARMA
26.3.1 COMPANY OVERVIEW
26.3.2 REVENUE ANALYSIS
26.3.3 GEOGRAPHIC PRESENCE
26.3.4 PRODUCT PORTFOLIO
26.3.5 RECENT DEVELOPMENTS
26.4 GLENMARK PHARMACEUTICALS LTD
26.4.1 COMPANY OVERVIEW
26.4.2 REVENUE ANALYSIS
26.4.3 GEOGRAPHIC PRESENCE
26.4.4 PRODUCT PORTFOLIO
26.4.5 RECENT DEVELOPMENTS
26.5 SANOFI-AVENTIS
26.5.1 COMPANY OVERVIEW
26.5.2 REVENUE ANALYSIS
26.5.3 GEOGRAPHIC PRESENCE
26.5.4 PRODUCT PORTFOLIO
26.5.5 RECENT DEVELOPMENTS
26.6 APOTEX INC.
26.6.1 COMPANY OVERVIEW
26.6.2 REVENUE ANALYSIS
26.6.3 GEOGRAPHIC PRESENCE
26.6.4 PRODUCT PORTFOLIO
26.6.5 RECENT DEVELOPMENTS
26.7 PFIZER INC.
26.7.1 COMPANY OVERVIEW
26.7.2 REVENUE ANALYSIS
26.7.3 GEOGRAPHIC PRESENCE
26.7.4 PRODUCT PORTFOLIO
26.7.5 RECENT DEVELOPMENTS
26.8 DR. REDDY’S LABORATORIES LTD.
26.8.1 COMPANY OVERVIEW
26.8.2 REVENUE ANALYSIS
26.8.3 GEOGRAPHIC PRESENCE
26.8.4 PRODUCT PORTFOLIO
26.8.5 RECENT DEVELOPMENTS
26.9 MERCK & CO., INC
26.9.1 COMPANY OVERVIEW
26.9.2 REVENUE ANALYSIS
26.9.3 GEOGRAPHIC PRESENCE
26.9.4 PRODUCT PORTFOLIO
26.9.5 RECENT DEVELOPMENTS
26.1 ELI LILLY AND COMPANY
26.10.1 COMPANY OVERVIEW
26.10.2 REVENUE ANALYSIS
26.10.3 GEOGRAPHIC PRESENCE
26.10.4 PRODUCT PORTFOLIO
26.10.5 RECENT DEVELOPMENTS
26.11 THE MENARINI GROUP
26.11.1 COMPANY OVERVIEW
26.11.2 REVENUE ANALYSIS
26.11.3 GEOGRAPHIC PRESENCE
26.11.4 PRODUCT PORTFOLIO
26.11.5 RECENT DEVELOPMENTS
26.12 ALVOGEN
26.12.1 COMPANY OVERVIEW
26.12.2 REVENUE ANALYSIS
26.12.3 GEOGRAPHIC PRESENCE
26.12.4 PRODUCT PORTFOLIO
26.12.5 RECENT DEVELOPMENTS
26.13 TABUK PHARMACEUTICA
26.13.1 COMPANY OVERVIEW
26.13.2 REVENUE ANALYSIS
26.13.3 GEOGRAPHIC PRESENCE
26.13.4 PRODUCT PORTFOLIO
26.13.5 RECENT DEVELOPMENTS
26.14 SPIMACO
26.14.1 COMPANY OVERVIEW
26.14.2 REVENUE ANALYSIS
26.14.3 GEOGRAPHIC PRESENCE
26.14.4 PRODUCT PORTFOLIO
26.14.5 RECENT DEVELOPMENTS
26.15 SUDAIR PHARMA
26.15.1 COMPANY OVERVIEW
26.15.2 REVENUE ANALYSIS
26.15.3 GEOGRAPHIC PRESENCE
26.15.4 PRODUCT PORTFOLIO
26.15.5 RECENT DEVELOPMENTS
26.16 LUPIN
26.16.1 COMPANY OVERVIEW
26.16.2 REVENUE ANALYSIS
26.16.3 GEOGRAPHIC PRESENCE
26.16.4 PRODUCT PORTFOLIO
26.16.5 RECENT DEVELOPMENTS
26.17 SUN PHARMA
26.17.1 COMPANY OVERVIEW
26.17.2 REVENUE ANALYSIS
26.17.3 GEOGRAPHIC PRESENCE
26.17.4 PRODUCT PORTFOLIO
26.17.5 RECENT DEVELOPMENTS
26.18 GLAXOSMITHKLINE
26.18.1 COMPANY OVERVIEW
26.18.2 REVENUE ANALYSIS
26.18.3 GEOGRAPHIC PRESENCE
26.18.4 PRODUCT PORTFOLIO
26.18.5 RECENT DEVELOPMENTS
26.19 TEVA PHARMACEUTICAL INDUSTRIES LTD
26.19.1 COMPANY OVERVIEW
26.19.2 REVENUE ANALYSIS
26.19.3 GEOGRAPHIC PRESENCE
26.19.4 PRODUCT PORTFOLIO
26.19.5 RECENT DEVELOPMENTS
26.2 ALKEM LABORATORIES LTD
26.20.1 COMPANY OVERVIEW
26.20.2 REVENUE ANALYSIS
26.20.3 GEOGRAPHIC PRESENCE
26.20.4 PRODUCT PORTFOLIO
26.20.5 RECENT DEVELOPMENTS
26.21 CIPLA
26.21.1 COMPANY OVERVIEW
26.21.2 REVENUE ANALYSIS
26.21.3 GEOGRAPHIC PRESENCE
26.21.4 PRODUCT PORTFOLIO
26.21.5 RECENT DEVELOPMENTS
26.22 ABBVIE INC.
26.22.1 COMPANY OVERVIEW
26.22.2 REVENUE ANALYSIS
26.22.3 GEOGRAPHIC PRESENCE
26.22.4 PRODUCT PORTFOLIO
26.22.5 RECENT DEVELOPMENTS
26.23 BAYER AG
26.23.1 COMPANY OVERVIEW
26.23.2 REVENUE ANALYSIS
26.23.3 GEOGRAPHIC PRESENCE
26.23.4 PRODUCT PORTFOLIO
26.23.5 RECENT DEVELOPMENTS
26.24 RADIUS HEALTH, INC.
26.24.1 COMPANY OVERVIEW
26.24.2 REVENUE ANALYSIS
26.24.3 GEOGRAPHIC PRESENCE
26.24.4 PRODUCT PORTFOLIO
26.24.5 RECENT DEVELOPMENTS
26.25 UCB S.A.
26.25.1 COMPANY OVERVIEW
26.25.2 REVENUE ANALYSIS
26.25.3 GEOGRAPHIC PRESENCE
26.25.4 PRODUCT PORTFOLIO
26.25.5 RECENT DEVELOPMENTS
26.26 DAIICHI SANKYO COMPANY
26.26.1 COMPANY OVERVIEW
26.26.2 REVENUE ANALYSIS
26.26.3 GEOGRAPHIC PRESENCE
26.26.4 PRODUCT PORTFOLIO
26.26.5 RECENT DEVELOPMENTS
26.27 VIATRIS INC.
26.27.1 COMPANY OVERVIEW
26.27.2 REVENUE ANALYSIS
26.27.3 GEOGRAPHIC PRESENCE
26.27.4 PRODUCT PORTFOLIO
26.27.5 RECENT DEVELOPMENTS
26.28 F. HOFFMANN-LA ROCHE LTD
26.28.1 COMPANY OVERVIEW
26.28.2 REVENUE ANALYSIS
26.28.3 GEOGRAPHIC PRESENCE
26.28.4 PRODUCT PORTFOLIO
26.28.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
27 RELATED REPORTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.