Global Osteosarcoma Market
Market Size in USD Million
CAGR :
% 
 USD
584.14 Million
USD
937.34 Million
2022
2023
USD
584.14 Million
USD
937.34 Million
2022
2023
| 2023 –2023 | |
| USD 584.14 Million | |
| USD 937.34 Million | |
|
|
|
|
Osteosarcoma Market Analysis and Size
Rising awareness amongst people is the vital factor escalating the market growth, also rise in research and development activities to launch a better treatment for osteosarcoma by leading players, rising government support for research and development, rising demand for new treatment of rare cancers such as osteosarcoma and increasing healthcare expenditure in some parts of the world are the major factors among others driving the acetaminophen (Paracetamol) market. Moreover, rising technological advancements and modernization in the healthcare devices, rising research and development activities in the healthcare sector and rising emerging markets with increasing geriatric population base will further create new opportunities for osteosarcoma market in the forecasted period.
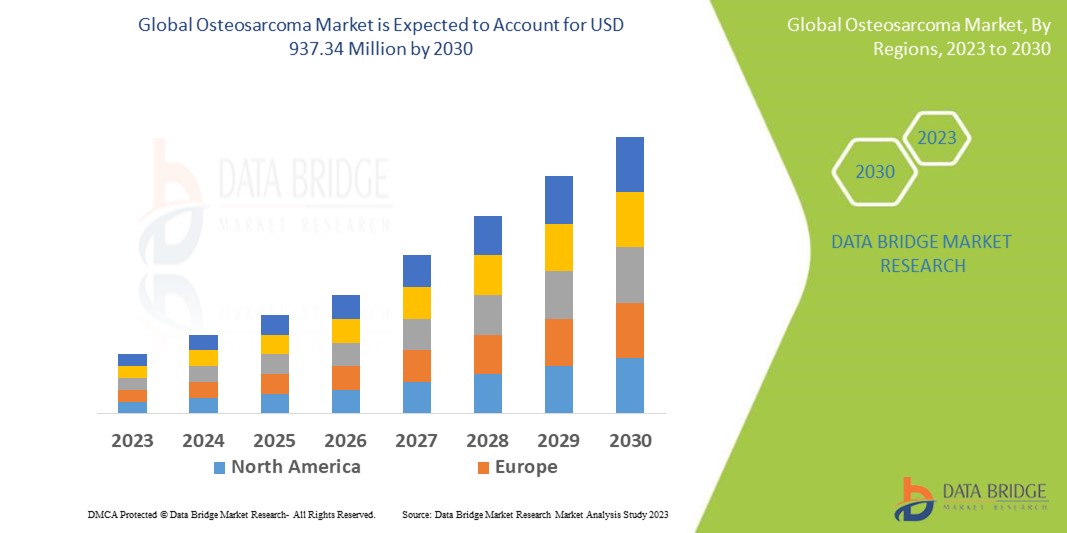
Data Bridge Market Research analyses that the osteosarcoma market which was USD 584.14 million in 2022, would rocket up to USD 937.34 million by 2030, and is expected to undergo a CAGR of 8.33% during the forecast period. This indicates that the market value. “Intramedullary Osteosarcoma” dominates the type segment of the Osteosarcoma market owing rising technological advancement. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Osteosarcoma Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Intramedullary Osteosarcoma, Juxtacortical Osteosarcoma, Extraskeletal Osteosarcoma), Diagnosis and Treatment (Treatment, Diagnosis), End-User (Hospitals and Clinics, Diagnostic Centers, Academic and Research Organizations) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America) |
|
Market Players Covered |
Pfizer Inc (U.S.), Mylan N.V. (U.S.), Novartis AG (Switzerland), Hikma Pharmaceuticals plc (U.K.), Aurobindo Pharma (India), Melinta Therapeutics, Inc (U.S.), Bristol-Myers Squibb Company (U.S.), GSK plc. (U.K.), Bayer AG (Germany), Teva Pharmaceuticals Industries Ltd. (Israel), Lilly (U.S.), AstraZeneca (U.K.), Amgen Inc. (U.S.), Bellicum Phamaceuticals, Inc (U.S.), Incyte (U.S.), Nektar Therapeutics (U.S.), Iovance Biotherapeutics (U.S.), Aurora biopharma (U.S.), BioAtla (U.S.), Eleison Pharmaceuticals (U.S.) and Acrotech Biopharma, Inc. (U.S.) among others) |
|
Market Opportunities |
|
Market Definition
Osteosarcoma or osteogenic sarcoma is the most common type of bone cancer, which develops in the cells that forms new bone tissues. Osteosarcoma most commonly occurs in the long bones of the body such as long bones of the hands or legs, although it can occur in any bone of the body. Symptoms of osteosarcoma include swelling and localized bone pain.
Global Osteosarcoma Market Dynamics
Drivers
- Increasing Incidence of Osteosarcoma
Osteosarcoma is one of the most common primary bone tumors, primarily affecting children and adolescents. The rising incidence of osteosarcoma globally is driving the demand for effective diagnosis, treatment, and management options
- Growing Focus on Precision Medicine
Precision medicine involves tailoring treatment strategies based on an individual's genetic profile, tumor characteristics, and overall health. The growing focus on precision medicine has led to the identification of specific genetic alterations and biomarkers in osteosarcoma, enabling targeted therapies and personalized treatment plans
- Innovations in Treatment Approaches
There have been significant advancements in the treatment of osteosarcoma, including surgical procedures, chemotherapy, targeted therapies, and radiation therapy. The development of novel treatment approaches, such as immunotherapy and personalized medicine, has shown promising results in improving survival rates and reducing treatment-related side effects
- Government Initiatives and Funding
Government initiatives aimed at improving cancer care, research funding, and healthcare infrastructure have contributed to the growth of the global osteosarcoma market. Increased funding allows for the development of new therapies, clinical trials, and the establishment of specialized centers for the diagnosis and treatment of osteosarcoma
Opportunities
- Rising Healthcare Expenditure
Increasing healthcare expenditure across the globe has positively impacted the osteosarcoma market. Higher healthcare spending allows for better infrastructure, improved access to healthcare services, and increased adoption of advanced treatment options, ultimately driving market growth
- Advancements in Diagnostic Technologies
Advances in diagnostic technologies, such as imaging techniques (X-ray, MRI, CT scans), molecular testing, and biopsy procedures, have improved the early detection and accurate diagnosis of osteosarcoma. These advancements have led to increased awareness, early intervention and improved patient outcomes
Restraints/Challenges
- High Cost of Treatment
The cost of osteosarcoma treatment can be substantial, including surgery, chemotherapy, radiation therapy, and supportive care. These expenses can place a significant financial burden on patients and their families. The high cost of treatment may limit access to advanced therapies and comprehensive care, particularly in low-income regions or countries with inadequate healthcare coverage
- Adverse Effects of Treatment
Osteosarcoma treatment, particularly chemotherapy and radiation therapy, can have significant side effects. These may include nausea, hair loss, fatigue, weakened immune system, and long-term complications such as organ damage. The potential for adverse effects can impact patient compliance with treatment regimens and quality of life
This osteosarcoma market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the osteosarcoma market Contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth
Recent Developments
- In December 2021, the Centers for Devices and Radiological Health of the FDA granted ZetaMet's breakthrough device for the treatment of metastatic bone cancers and osteological interventions
- In October 2020, Johnson & Johnson completed the acquisition of Momenta Pharmaceuticals Inc. acquisition to provide additional potential in various diseases, including infectious diseases and vaccines, neuroscience, oncology and pulmonary hypertension
Global Osteosarcoma Market Scope
The osteosarcoma market is segmented on the basis of type, diagnosis and treatment and end-user. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Intramedullary Osteosarcoma
- Juxtacortical Osteosarcoma
- Extraskeletal Osteosarcoma
Diagnosis and Treatment
- Treatment
- Diagnosis
End-User
- Hospitals and Clinics
- Diagnostic Centers
- Academic and Research Organizations
Osteosarcoma Market Regional Analysis/Insights
The osteosarcoma market is analysed and market size insights and trends are provided by country, type, diagnosis and treatment and end-user as referenced above.
The countries covered in the osteosarcoma market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
North America dominates the osteosarcoma market because of the strong base of healthcare facilities, strong presence of major players in the market, increasing incidence of osteosarcoma and rising number of research activities in this region.
Asia-Pacific is expected to witness significant growth during the forecast period from 2023 to 2030 due to the increase in government initiatives to promote awareness, rise in medical tourism, growing research activities in the region, availability of massive untapped markets, large population pool, and the growing demand for quality healthcare in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and up-stream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Osteosarcoma Market Share Analysis
The osteosarcoma market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the company’s focus related to osteosarcoma market.
Some of the major players operating in the osteosarcoma market are
- Pfizer Inc (U.S.)
- Mylan N.V. (U.S.)
- Novartis AG (Switzerland.)
- Hikma Pharmaceuticals plc (U.K.)
- Aurobindo Pharma (India)
- Melinta Therapeutics, Inc (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- GSK plc. (U.K.)
- Bayer AG (Germany)
- Teva Pharmaceuticals Industries Ltd. (Israel)
- Lilly (U.S.)
- Amgen Inc. (U.S.)
- Bellicum Phamaceuticals, Inc (U.S.)
- Incyte (U.S.)
- Nektar Therapeutics (U.S.)
- Iovance Biotherapeutics (U.S.)
- Aurora biopharma (U.S.)
- BioAtla (U.S.)
- Acrotech Biopharma, Inc. (U.S.)
- Eleison Pharmaceuticals (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL OSTEOSARCOMA MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL XX SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL OSTEOSARCOMA MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR GLOBAL OSTEOSARCOMA TREATMENT MARKET
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR GLOBAL OSTEOSARCOMA TREATMENT MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
10.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.13.1 FORECAST MARKET OUTLOOK
10.13.2 CROSS COMPETITION
10.13.3 THERAPEUTIC PORTFOLIO
10.13.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 MARKET OVERVIEW
13.1 DRIVERS
13.2 RESTRAINTS
13.3 OPPORTUNITIES
13.4 CHALLENGES
14 GLOBAL OSTEOSARCOMA TREATMENT MARKET, SWOT AND DBMR ANALYSIS
15 GLOBAL OSTEOSARCOMA MARKET, BY TREATMENT & DIAGNOSIS
15.1 OVERVIEW
15.2 TREATMENT
15.2.1 MEDICATION
15.2.1.1. MARKETED DRUGS
15.2.1.1.1. CHEMOTHERAPY
15.2.1.1.1.1 METHOTREXATE
15.2.1.1.1.2 DOXORUBICIN (ADRIAMYCIN)
15.2.1.1.1.3 IFSOSFAMIDE
15.2.1.1.1.4 ETOPOSIDE
15.2.1.1.1.5 GEMCITABINE
15.2.1.1.1.6 OTHERS
15.2.1.1.2. TARGETED THERAPY
15.2.1.1.2.1 KINASE INHIBITOR THERAPY
15.2.1.1.2.1.1. CABOZANTINIB
15.2.1.1.2.1.2. REGORAFENIB
15.2.1.1.2.1.3. OTHERS
15.2.1.1.2.2 MAMMALIAN TARGET OF RAPAMYCIN (MTOR) INHIBITORS
15.2.1.1.2.2.1. EVEROLIMUS
15.2.1.1.2.2.2. OTHERS
15.2.1.1.3. SAMARIUM
15.2.1.1.3.1 LEXIDRONAM
15.2.1.1.3.2 OTHERS
15.2.1.2. EMERGING/PIPELINE DRUGS
15.2.1.2.1. CHEMOTHERAPY
15.2.1.2.1.1 CISPLASTIN
15.2.1.2.1.2 PAMIDRONATE
15.2.1.2.1.3 SURUFATINIB
15.2.1.2.1.4 ENDOSTAR
15.2.1.2.1.5 OTHERS
15.2.1.2.2. TARGETED THERAPY
15.2.1.2.2.1 PAZOPANIB
15.2.1.2.2.2 OTHERS
15.2.1.2.3. IMMUNOTHERAPY
15.2.1.2.3.1 NIVOLUMAB
15.2.1.2.3.2 OTHERS
15.2.1.2.4. KINASE INHIBITORS
15.2.1.2.4.1 ALIQOPA
15.2.1.2.4.2 REGORAFENIB
15.2.1.2.4.3 CABOZANTINIB S-MALATE
15.2.1.2.4.4 OTHERS
15.2.2 RADIATION THERAPY
15.2.2.1. EXTERNAL RADIATION THERAPY
15.2.2.1.1. INTENSITY MODULATED RADIATION THERAPY (IMRT)
15.2.2.1.2. CONFORMAL RADIATION BEAM THERAPY
15.2.2.1.3. STEREOTACTIC RADIOSURGERY
15.2.2.1.4. OTHERS
15.2.2.2. INTERNAL RADIATION THERAPY
15.2.2.2.1. BRACHYTHERAPY
15.2.2.2.2. OTHERS
15.2.3 SURGERY
15.2.3.1. LIMB-SPARING SURGERY
15.2.3.2. AMPUTATION
15.2.3.3. ROTATIONPLASTY
15.2.3.4. WIDE LOCAL EXCISION
15.2.3.5. METASTASECTOMY
15.3 DIAGNOSIS
15.3.1 IMAGING
15.3.1.1. COMPUTED TOMOGRAPHY (CT)
15.3.1.2. MAGNETIC RESONANCE IMAGING (MRI)
15.3.1.3. X-RAY
15.3.1.4. POSITRON EMISSION TOMOGRAPHY (PET)
15.3.1.5. OTHERS
15.3.2 BIOPSIES
15.3.2.1. CORE BIOPSY
15.3.2.2. INCISIONAL BIOPSY
15.3.3 BLOOD TEST
15.3.3.1. COMPLETE BLOOD TEST (CBC)
15.3.3.2. BLOOD CHEMISTRY TESTS
16 GLOBAL OSTEOSARCOMA MARKET, BY TYPE
16.1 OVERVIEW
16.2 CENTRAL TUMOR (MEDULLARY)
16.2.1 CONVENTIONAL CENTRAL OSTEOSARCOMA
16.2.2 TELANGIECTATIC OSTEOSARCOMA
16.2.3 INTRAOSSEOUS WELL-DIFFERENTIATED OSREOSARCOMA
16.2.4 SMALL CELL OSTEOSARCOMA
16.3 SURFACE TUMOR (PERIPHERAL)
16.3.1 PAROSTEAL WELL-DIFFERENTIATED
16.3.2 PERIOSTEAL OSTEOSARCOMA
16.3.3 HIGH-GRADE SURFACE OSTEOSARCOMA
17 GLOBAL OSTEOSARCOMA MARKET, BY STAGES
17.1 OVERVIEW
17.2 LOW-GRAD
17.2.1 PAROSTEAL (JUXTACORTIAL LOW GRADE).
17.2.2 INTRAMEDULLARY OR INTRAOSSEOUS WELL-DIFFERENTIATED (LOW-GRADE CENTRAL)
17.3 HIGH-GRADE
17.3.1 OSTEOBLASTIC.
17.3.2 CHONDROBLASTIC.
17.3.3 FIBROBLASTIC.
17.3.4 SMALL CELL.
17.3.5 TELANGIECTATIC.
17.3.6 HIGH-GRADE SURFACE (JUXTACORTICAL HIGH-GRADE).
17.3.7 PAGETOID.
17.3.8 EXTRASKELETAL.
17.3.9 POST-RADIATION.
17.4 INTERMEDIATE GRADE (JUXTACORTICAL)
18 GLOBAL OSTEOSARCOMA MARKET, BY ROUTE OF ADMINISTRATION
18.1 OVERVIEW
18.2 PARENTERAL
18.3 ORAL
18.4 OTHERS
19 GLOBAL OSTEOSARCOMA MARKET, BY DOSAGE FORM
19.1 OVERVIEW
19.2 ORAL
19.2.1 PILLS
19.2.2 TABLETS
19.2.3 CAPSULES
19.2.4 OTHERS
19.3 INTRAVENOUS
19.4 SUBCUTANOUS
19.5 OTHERS
20 GLOBAL OSTEOSARCOMA MARKET, BY AGE GROUP
20.1 OVERVIEW
20.2 ADULT
20.3 GERIARTIC
20.4 PEDIATRIC
21 GLOBAL OSTEOSARCOMA MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS & CLINICS
21.3 DIAGNOSTIC CENTERS
21.4 ACADEMIC & RESEARCH INSTITUTES
21.5 OTHERS
22 GLOBAL OSTEOSARCOMA MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDER
22.3 RETAIL SALES
22.3.1 HOSPITAL PHARMACIES
22.3.2 ONLINE PHARMACIES
22.3.3 OTHERS
22.4 OTHERS
23 GLOBAL OSTEOSARCOMA TREATMENT MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: GLOBAL
23.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS: EUROPE
23.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.5 MERGERS & ACQUISITIONS
23.6 NEW PRODUCT DEVELOPMENT & APPROVALS
23.7 EXPANSIONS
23.8 REGULATORY CHANGES
23.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL OSTEOSARCOMA TREATMENT MARKET, BY REGION
GLOBAL OSTEOSARCOMA TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.1 NORTH AMERICA
24.1.1 U.S.
24.1.2 CANADA
24.1.3 MEXICO
24.2 EUROPE
24.2.1 GERMANY
24.2.2 U.K.
24.2.3 ITALY
24.2.4 FRANCE
24.2.5 SPAIN
24.2.6 RUSSIA
24.2.7 SWITZERLAND
24.2.8 TURKEY
24.2.9 BELGIUM
24.2.10 NETHERLANDS
24.2.11 DENMARK
24.2.12 SWEDEN
24.2.13 POLAND
24.2.14 NORWAY
24.2.15 FINLAND
24.2.16 REST OF EUROPE
24.3 ASIA-PACIFIC
24.3.1 JAPAN
24.3.2 CHINA
24.3.3 SOUTH KOREA
24.3.4 INDIA
24.3.5 SINGAPORE
24.3.6 THAILAND
24.3.7 INDONESIA
24.3.8 MALAYSIA
24.3.9 PHILIPPINES
24.3.10 AUSTRALIA
24.3.11 NEW ZEALAND
24.3.12 VIETNAM
24.3.13 TAIWAN
24.3.14 REST OF ASIA-PACIFIC
24.4 SOUTH AMERICA
24.4.1 BRAZIL
24.4.2 ARGENTINA
24.4.3 REST OF SOUTH AMERICA
24.5 MIDDLE EAST AND AFRICA
24.5.1 SOUTH AFRICA
24.5.2 EGYPT
24.5.3 BAHRAIN
24.5.4 UNITED ARAB EMIRATES
24.5.5 KUWAIT
24.5.6 OMAN
24.5.7 QATAR
24.5.8 SAUDI ARABIA
24.5.9 REST OF MEA
24.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 GLOBAL OSTEOSARCOMA TREATMENT MARKET, COMPANY PROFILE
25.1 CELLECTAR BIOSCIENCES, INC
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 NOVARTIS
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 PFIZER INC.
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 HIKMA PHARMACEUTICAL
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 SPECTRUM PHARMACEUTICAL
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 ASTRAZENECA
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 ZENTALIS PHARMACEUTICALS
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 GSK
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 SAM BIOSCIENCES
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 RADIOPHARM THERANOSTICS
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 ISOFOL MEDICAL AB
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 ADVAXIS, INC
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.