Global Peripheral Arterial Disease Pad Market
Market Size in USD Million
CAGR :
% 
 USD
873.01 Million
USD
1.74 Million
2024
2032
USD
873.01 Million
USD
1.74 Million
2024
2032
| 2025 –2032 | |
| USD 873.01 Million | |
| USD 1.74 Million | |
|
|
|
|
Peripheral Arterial Disease (PAD) Market Size
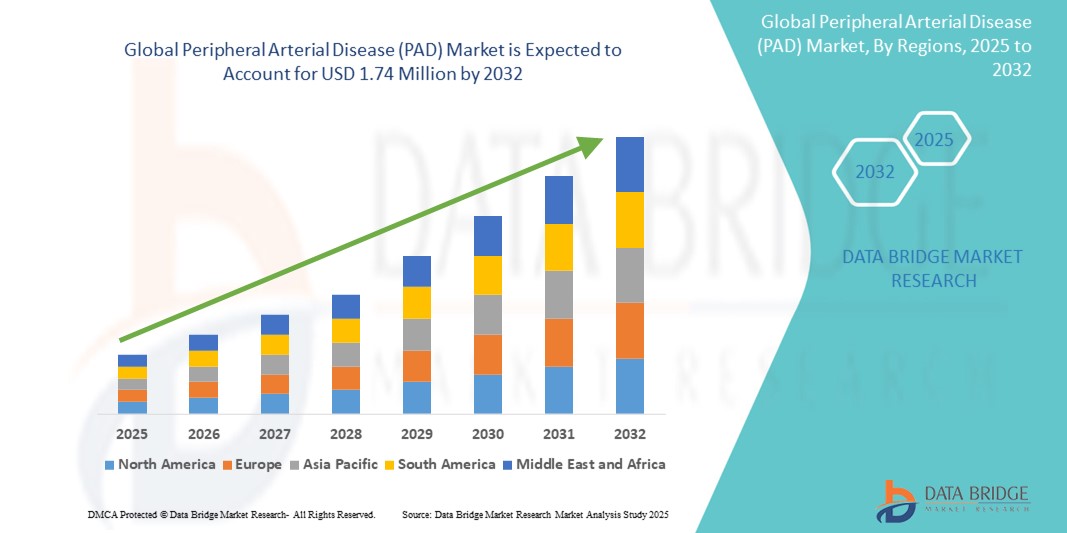
- The global peripheral arterial disease (PAD) market was valued at USD 873.01 million in 2024 and is expected to reach USD 1.74 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 9.00%, primarily driven by the increasing prevalence of PAD due to aging populations, rising risk factors such as diabetes and hypertension, and advancements in minimally invasive treatment options
- This growth is driven by factors such as the rising incidence of cardiovascular diseases, increased awareness about PAD, advancements in diagnostic technologies, and the development of innovative treatment options such as drug-eluting stents and atherectomy devices
Peripheral Arterial Disease (PAD) Market Analysis
- Peripheral arterial disease (PAD) is a common circulatory problem where narrowed arteries reduce blood flow to the limbs, particularly affecting the lower extremities. PAD treatments include medications, angioplasty, stenting, and surgical interventions
- The demand for PAD treatments is largely driven by the growing prevalence of risk factors such as aging, diabetes, hypertension, and smoking. With the aging global population and increasing chronic conditions, the need for effective PAD management continues to rise
- North America is a dominant region for the PAD market, driven by advanced healthcare infrastructure, high awareness of cardiovascular diseases, and a significant population suffering from risk factors such as diabetes and hypertension. The region is also a leader in adopting new technologies and treatment methods for PAD
- For instance, in the United States, the prevalence of Peripheral Arterial Disease (PAD) is rising, particularly among individuals over the age of 60, due to the aging population and increasing rates of diabetes and hypertension.
- Globally, PAD treatments are considered among the most crucial interventions in cardiovascular care, with a growing focus on minimally invasive techniques such as endovascular surgery, which ensure faster recovery times and better outcomes for patients
Report Scope and Peripheral Arterial Disease (PAD) Market Segmentation
|
Attributes |
Peripheral Arterial Disease (PAD) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Peripheral Arterial Disease (PAD) Market Trends
“Adoption of Minimally Invasive Treatment Technologies”
- A prominent trend in the global peripheral arterial disease market is the increasing adoption of minimally invasive treatment technologies, such as endovascular procedures, atherectomy, and drug-eluting stents
- These advanced techniques provide patients with less invasive alternatives to traditional surgery, resulting in shorter recovery times, reduced complications, and improved overall outcomes.
- For instance, endovascular revascularization techniques, such as angioplasty and stenting, allow for targeted treatment of blocked arteries with smaller incisions, enabling faster recovery and less post-operative pain compared to conventional open surgeries
- These technologies also contribute to the development of more personalized treatment options, improving the precision and effectiveness of PAD management
- This trend is transforming the PAD treatment landscape, increasing patient demand for less invasive, quicker recovery solutions and driving the growth of the global PAD market
Peripheral Arterial Disease (PAD) Market Dynamics
Driver
“Increasing Prevalence of Risk Factors and Aging Population”
- The rising prevalence of risk factors such as diabetes, hypertension, smoking, and high cholesterol, combined with the aging global population, is significantly driving the demand for PAD treatments
- As people age, the likelihood of developing PAD increases, particularly in individuals with comorbid conditions such as diabetes, which accelerates the progression of arterial plaque buildup and narrowing of blood vessels
- PAD is one of the most common causes of limb amputation, and early intervention is crucial for preventing severe complications. This has heightened the demand for advanced diagnostic tools and effective treatment options
- The growing awareness of PAD, coupled with the aging population, is driving the need for more specialized procedures and medical equipment aimed at diagnosing and treating PAD, such as angioplasty devices and stents.
- As healthcare systems prioritize managing chronic diseases and improving cardiovascular health, the demand for advanced PAD treatment technologies continues to rise, ensuring better patient outcomes and reducing long-term healthcare costs
For instance,
- In 2021, the American Heart Association reported that more than 8.5 million people in the U.S. aged 40 and older suffer from PAD, and the prevalence is expected to rise as the population ages, directly contributing to the growing demand for PAD treatments and related technologies.
- In 2020, the World Health Organization noted that by 2050, 16% of the global population will be over 65, further highlighting the rising need for effective PAD management and driving the growth of the PAD market
- As a result of the increasing prevalence of risk factors and the aging population, the demand for PAD treatments and diagnostic technologies continues to grow, enhancing market growth and innovation.
Opportunity
“Integration of Artificial Intelligence and Digital Technologies”
- AI and digital technologies offer significant opportunities to enhance the diagnosis, treatment, and management of PAD by improving precision and efficiency in both clinical settings and surgery
- AI-powered systems can analyze diagnostic imaging such as angiograms, CT scans, and MRIs in real-time, helping clinicians identify arterial blockages, stenosis, and other abnormalities with high accuracy. This aids in earlier detection, which is crucial for preventing severe complications such as limb amputation
- In addition, AI algorithms can assist in predictive analytics, enabling healthcare providers to identify high-risk patients and monitor disease progression, leading to more personalized treatment plans and better patient outcomes
For instance,
- In January 2024, a study published in the Journal of Vascular Surgery demonstrated that AI systems could accurately analyze CT angiography images to detect PAD with higher sensitivity than traditional methods, potentially leading to earlier interventions and improved patient prognosis
- In December 2023, a partnership between a leading medical device manufacturer and an AI startup was announced, focusing on integrating machine learning algorithms into stent deployment systems. This innovation aims to improve the precision of stent placement during PAD procedures, minimizing complications and enhancing long-term results
- The integration of AI in PAD treatment is expected to optimize procedural planning, reduce human error, and improve recovery times. AI-powered systems can also assist clinicians in choosing the most effective treatments and monitoring patients over time, opening new avenues for personalized care and improved long-term management of PAD
Restraint/Challenge
“High Treatment and Equipment Costs Hindering Widespread Adoption”
- The high costs associated with PAD treatments and diagnostic equipment present a significant challenge, particularly in low- and middle-income countries. The cost of advanced diagnostic devices such as angiography systems and atherectomy tools, as well as procedures such as stenting and endovascular surgeries, can be prohibitively expensive for many healthcare providers
- These high costs can deter hospitals and clinics, especially those in developing regions, from adopting the latest PAD treatment technologies, limiting the accessibility of advanced care for PAD patients
- The financial burden of these treatments may also place pressure on patients, leading to delayed diagnoses and treatments, which can result in severe complications such as limb amputation or worsening cardiovascular health
For instance,
- In August 2024, a report published by the International Journal of Cardiology highlighted that the high cost of angioplasty and stenting procedures is a major barrier to PAD treatment in developing nations, limiting access to timely interventions and contributing to poorer health outcomes
- Consequently, the high treatment and equipment costs can create disparities in the quality of care and access to advanced PAD management, hindering the market's overall growth and the widespread adoption of innovative PAD treatments and technologies.
Peripheral Arterial Disease (PAD) Market Scope
The market is segmented on the basis of treatment type, route of administration, end user and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Treatment Type |
|
|
By Route of Administration |
|
|
By End User |
|
|
By Distribution Channel
|
|
Peripheral Arterial Disease (PAD) Market Regional Analysis
“North America is the Dominant Region in the Peripheral Arterial Disease (PAD) Market”
- North America holds a dominant share of the global PAD market, driven by its advanced healthcare infrastructure, high awareness of cardiovascular diseases, and early adoption of cutting-edge medical technologies
- U.S. plays a crucial role in this dominance due to its well-established healthcare system, rising prevalence of PAD, and strong focus on cardiovascular health. With an aging population and a growing number of PAD cases, the demand for diagnostic tools and treatment options continues to rise
- The region benefits from high investment in research & development by leading medical device companies, ensuring the availability of the latest treatment methods, including minimally invasive procedures such as angioplasty and stent placement
- In addition, the reimbursement policies in the U.S. contribute significantly to the accessibility of PAD treatments, further driving market growth
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is expected to register the highest growth rate in the PAD market, driven by rapid improvements in healthcare infrastructure, rising awareness about vascular diseases, and increasing prevalence of risk factors such as diabetes and hypertension
- Countries such as China, India, and Japan are emerging as key markets due to their large populations, increasing cases of diabetes and cardiovascular diseases, and aging demographics. These nations are focusing on enhancing healthcare services to address the growing burden of PAD.
- Japan, with its advanced healthcare system and aging population, remains a significant market for PAD diagnostics and treatments, offering opportunities for the adoption of advanced treatment technologies such as drug-eluting stents and atherectomy devices
- In China and India, rising government and private sector investments in healthcare infrastructure and the expansion of PAD treatment options are contributing to the growing demand for innovative PAD therapies, positioning the region for substantial market growth
Peripheral Arterial Disease (PAD) Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Bayer AG (Germany)
- Abbott (U.S.)
- Boston Scientific Corporation (U.S.)
- Biotronik (Germany)
- Cardinal Health (U.S.)
- Terumo Corporation (Japan)
- AngioDynamics (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- Medtronic (Ireland)
- B. Braun SE (Germany)
- iVascular (Spain)
- Endologix LLC (U.S.)
- BD (U.S.)
- Meril Life Sciences Pvt. Ltd. (India)
- Lombard Medical (U.K.)
- Shockwave Medical Inc. (U.S.)
- Cordis (U.S.)
- Penumbra, Inc. (U.S.)
- BIOCARDIA, INC. (U.S.)
- Heraeus Group (Germany)
Latest Developments in Global Peripheral Arterial Disease (PAD) Market
- In March 2024, Becton, Dickinson and Company (BD) launched the AGILITY clinical study to evaluate the safety and effectiveness of its Vascular Covered Stent in treating PAD. The study is being conducted internationally across the U.S., Europe, Australia, and New Zealand, with the goal of expanding treatment options for PAD
- In May 2024, The American Heart Association (AHA) published updated guidelines that emphasize early diagnosis, structured exercise therapy, and coordinated multispecialty care to reduce the risk of amputations in PAD patients. These guidelines also highlight racial and ethnic disparities in PAD outcomes.
- In May 2024, The American Heart Association introduced a comprehensive roadmap that emphasizes early diagnosis, structured exercise, and coordinated care to mitigate the risk of amputations for PAD patients. This initiative focuses on reducing healthcare disparities and improving patient outcomes.
- In March 2024, BD announced the enrollment of the first patient in its AGILITY study, a global clinical trial assessing the safety and effectiveness of its Vascular Covered Stent for PAD treatment. The goal is to offer a new solution for interventional treatments of PAD
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
6 EPIDEMIOLOGY
7 INDUSTRY INSIGHTS
8 REGULATORY FRAMEWORK
9 PIPELINE ANALYSIS
9.1 PHASE III CANDIDATES
9.2 PHASE II CANDIDATES
9.3 PHASE I CANDIDATES
9.4 OTHERS (PRE-CLINICAL AND RESEARCH)
10 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, BY TREATMENT TYPE
10.1 OVERVIEW
10.1.1 THERAPEUTIC DRUGS
10.1.1.1. CHOLESTEROL-LOWERING MEDICATIONS
10.1.1.1.1. WARFARIN
10.1.1.1.2. HEPARIN
10.1.1.1.3. STATINS
10.1.1.1.3.1 ATORVASTATIN
10.1.1.1.3.2 FLUVASTATIN
10.1.1.1.3.3 LOVASTATIN
10.1.1.1.3.4 PITAVASTATIN
10.1.1.1.3.5 SIMVASTATIN
10.1.1.1.3.6 OTHERS
10.1.1.2. BLOOD-PRESSURE LOWERING
10.1.1.2.1. ANGIOTENSIN-CONVERTING ENZYME (ACE) INHIBITORS
10.1.1.2.1.1 BENAZEPRIL
10.1.1.2.1.2 CAPTOPRIL
10.1.1.2.1.3 ENALAPRIL
10.1.1.2.1.4 MOEXIPRIL
10.1.1.2.1.5 PERINDOPRIL
10.1.1.2.1.6 OTHERS
10.1.1.2.2. BETA BLOCKERS
10.1.1.2.2.1 NEBIVOLOL
10.1.1.2.2.2 METOPROLOL SUCCINATE
10.1.1.2.2.3 OTHERS
10.1.1.3. BLOOD SUGAR CONTROL
10.1.1.3.1. DPP-4 INHIBITORS
10.1.1.3.1.1 SAXAGLIPTIN
10.1.1.3.1.2 SITAGLIPTIN
10.1.1.3.1.3 LINAGLIPTIN
10.1.1.3.1.4 OTHERS
10.1.1.3.2. GLP-1 RECEPTOR AGONISTS
10.1.1.3.2.1 EXENATIDE
10.1.1.3.2.2 LIRAGLUTIDE
10.1.1.3.2.3 ALBIGLUTIDE
10.1.1.3.2.4 OTHERS
10.1.1.4. ANTIPLATELET MEDICINES
10.1.1.4.1. ASPIRIN THERAPY
10.1.1.4.2. CLOPIDOGREL
10.1.1.4.3. TICLOPIDINE
10.1.1.4.4. DIPYRIDAMOLE
10.1.1.4.5. OTHERS
10.1.1.5. SYMPTOM-RELIEF MEDICATIONS
10.1.1.5.1. CILOSTAZOL
10.1.1.5.2. PENTOXIFYLLINE
10.1.1.5.3. OTHERS
10.1.2 THERAPUETIC DEVICES
10.1.2.1. PERIPHERAL VASCULAR STENTS
10.1.2.1.1. SELF-EXPANDABLE
10.1.2.1.2. BALLOON-EXPANDABLE
10.1.2.1.3. COVERED
10.1.2.1.4. DRUG-ELUTING STENTS
10.1.2.2. PERCUTANEOUS TRANSLUMINAL ANGIOPLASTY (PTA) BALLOON CATHETERS
10.1.2.2.1. STANDARD PTA BALLOON
10.1.2.2.2. HIGH PRESSURE PTA BALLOON
10.1.2.2.3. SCORING PTA BALLOON
10.1.2.2.4. CUTTING BALLOONS
10.1.2.2.5. DRUG COATED BALLOON
10.1.2.2.6. OTHERS
10.1.2.3. PERIPHERAL TRANSLUMINAL ANGIOPLASTY (PTA) GUIDEWIRES
10.1.2.3.1. COATED PTA GUIDEWIRES
10.1.2.3.2. NON-COATED PTA GUIDEWIRES
10.1.2.4. ATHERECTOMY DEVICES
10.1.2.4.1. ORBITAL ATHERECTOMY DEVICES
10.1.2.4.2. ROTATIONAL ATHERECTOMY DEVICES
10.1.2.4.3. LASER ATHERECTOMY DEVICES
10.1.2.4.4. DIRECTIONAL ATHERECTOMY DEVICES
10.1.2.5. EMBOLIC PROTECTION DEVICES
10.1.2.5.1. DISTAL OCCLUSION ASPIRATION DEVICES
10.1.2.5.2. DISTAL FILTERS ASPIRATION DEVICES
10.1.2.5.3. PROXIMAL OCCLUSION ASPIRATION DEVICES
10.1.2.6. INFERIOR VENA CAVA FILTERS
10.1.2.6.1. PERMANENT INFERIOR VENA CAVA FILTERS
10.1.2.6.2. RETREIVABLE INFERIOR VENA CAVA FILTERS
10.1.2.7. OTHER DEVICES
11 GLOBAL MEDICATION IN PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, BY MODE OF PRESCRIPTION
11.1 OVERVIEW
11.2 PRESCRIPTION
11.3 OVER THE COUNTER (OTC)
12 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, BY TYPE
12.1 OVERVIEW
12.2 FUNCTIONAL PERIPHERAL ARTERIAL DISEASE PAD
12.3 ORGANIC PERIPHERAL ARTERIAL DISEASE PAD
13 GLOBAL MEDICATION IN PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 ORAL
13.2.1 TABLET
13.2.2 CAPSULE
13.2.3 OTHERS
13.3 PARENTERAL
13.3.1 INTRAVENOUS
13.3.2 SUBCUTANEOUS
13.3.3 OTHERS
14 GLOBAL MEDICATION IN PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, BY AGE
14.1 OVERVIEW
14.2 CHILDREN
14.3 ADULT
14.4 GERIATRIC
15 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITALS
15.2.1 PUBLIC
15.2.2 PRIVATE
15.3 CATH LABORATORY
15.4 SPECIALTY CLINICS
15.5 AMBULATORY SURGICAL CENTERS
15.6 HOME HEALTHCARE
15.7 OTHERS
16 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL PHARMACY
16.3.1 HOSPITAL PHARMACY
16.3.2 RETAIL PHARMACY
16.3.3 ONLINE PHARMACY
16.3.4 OTHERS
16.4 OTHERS
17 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: GLOBAL
17.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
17.3 COMPANY SHARE ANALYSIS: EUROPE
17.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
17.5 MERGERS & ACQUISITIONS
17.6 NEW PRODUCT DEVELOPMENT & APPROVALS
17.7 EXPANSIONS
17.8 REGULATORY CHANGES
17.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
18 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, BY GEOGRAPHY
18.1 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
18.1.1 NORTH AMERICA
18.1.1.1. U.S.
18.1.1.2. CANADA
18.1.1.3. MEXICO
18.1.2 EUROPE
18.1.2.1. GERMANY
18.1.2.2. FRANCE
18.1.2.3. U.K.
18.1.2.4. HUNGARY
18.1.2.5. LITHUANIA
18.1.2.6. AUSTRIA
18.1.2.7. IRELAND
18.1.2.8. NORWAY
18.1.2.9. POLAND
18.1.2.10. ITALY
18.1.2.11. SPAIN
18.1.2.12. RUSSIA
18.1.2.13. TURKEY
18.1.2.14. NETHERLANDS
18.1.2.15. SWITZERLAND
18.1.2.16. REST OF EUROPE
18.1.3 ASIA-PACIFIC
18.1.3.1. JAPAN
18.1.3.2. CHINA
18.1.3.3. SOUTH KOREA
18.1.3.4. INDIA
18.1.3.5. AUSTRALIA
18.1.3.6. SINGAPORE
18.1.3.7. THAILAND
18.1.3.8. MALAYSIA
18.1.3.9. INDONESIA
18.1.3.10. PHILIPPINES
18.1.3.11. VIETNAM
18.1.3.12. REST OF ASIA-PACIFIC
18.1.4 SOUTH AMERICA
18.1.4.1. BRAZIL
18.1.4.2. ARGENTINA
18.1.4.3. PERU
18.1.4.4. REST OF SOUTH AMERICA
18.1.5 MIDDLE EAST AND AFRICA
18.1.5.1. SOUTH AFRICA
18.1.5.2. SAUDI ARABIA
18.1.5.3. UAE
18.1.5.4. EGYPT
18.1.5.5. ISRAEL
18.1.5.6. REST OF MIDDLE EAST AND AFRICA
18.1.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
19 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, SWOT AND DBMR ANALYSIS
20 GLOBAL PERIPHERAL ARTERIAL DISEASE (PAD) MARKET, COMPANY PROFILE
20.1 ABBOTT.
20.1.1 COMPANY OVERVIEW
20.1.2 REVENUE ANALYSIS
20.1.3 GEOGRAPHIC PRESENCE
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.2 KONINKLIJKE PHILIPS N.V.
20.2.1 COMPANY OVERVIEW
20.2.2 REVENUE ANALYSIS
20.2.3 GEOGRAPHIC PRESENCE
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 B. BRAUN SE
20.3.1 COMPANY OVERVIEW
20.3.2 REVENUE ANALYSIS
20.3.3 GEOGRAPHIC PRESENCE
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENTS
20.4 BIOTRONIK SE & CO. KG
20.4.1 COMPANY OVERVIEW
20.4.2 REVENUE ANALYSIS
20.4.3 GEOGRAPHIC PRESENCE
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENTS
20.5 BOSTON SCIENTIFIC CORPORATION
20.5.1 COMPANY OVERVIEW
20.5.2 REVENUE ANALYSIS
20.5.3 GEOGRAPHIC PRESENCE
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENTS
20.6 COOK
20.6.1 COMPANY OVERVIEW
20.6.2 REVENUE ANALYSIS
20.6.3 GEOGRAPHIC PRESENCE
20.6.4 PRODUCT PORTFOLIO
20.6.5 RECENT DEVELOPMENTS
20.7 TERUMO EUROPE NV
20.7.1 COMPANY OVERVIEW
20.7.2 REVENUE ANALYSIS
20.7.3 GEOGRAPHIC PRESENCE
20.7.4 PRODUCT PORTFOLIO
20.7.5 RECENT DEVELOPMENTS
20.8 JANSSEN PHARMACEUTICALS, INC.
20.8.1 COMPANY OVERVIEW
20.8.2 REVENUE ANALYSIS
20.8.3 GEOGRAPHIC PRESENCE
20.8.4 PRODUCT PORTFOLIO
20.8.5 RECENT DEVELOPMENTS
20.9 OTSUKA PHARMACEUTICAL CO., LTD.
20.9.1 COMPANY OVERVIEW
20.9.2 REVENUE ANALYSIS
20.9.3 GEOGRAPHIC PRESENCE
20.9.4 PRODUCT PORTFOLIO
20.9.5 RECENT DEVELOPMENTS
20.1 BAYER AG
20.10.1 COMPANY OVERVIEW
20.10.2 REVENUE ANALYSIS
20.10.3 GEOGRAPHIC PRESENCE
20.10.4 PRODUCT PORTFOLIO
20.10.5 RECENT DEVELOPMENTS
20.11 MEDTRONIC
20.11.1 COMPANY OVERVIEW
20.11.2 REVENUE ANALYSIS
20.11.3 GEOGRAPHIC PRESENCE
20.11.4 PRODUCT PORTFOLIO
20.11.5 RECENT DEVELOPMENTS
20.12 MYLAN N.V.
20.12.1 COMPANY OVERVIEW
20.12.2 REVENUE ANALYSIS
20.12.3 GEOGRAPHIC PRESENCE
20.12.4 PRODUCT PORTFOLIO
20.12.5 RECENT DEVELOPMENTS
20.13 ANGIODYNAMICS
20.13.1 COMPANY OVERVIEW
20.13.2 REVENUE ANALYSIS
20.13.3 GEOGRAPHIC PRESENCE
20.13.4 PRODUCT PORTFOLIO
20.13.5 RECENT DEVELOPMENTS
20.14 BD
20.14.1 COMPANY OVERVIEW
20.14.2 REVENUE ANALYSIS
20.14.3 GEOGRAPHIC PRESENCE
20.14.4 PRODUCT PORTFOLIO
20.14.5 RECENT DEVELOPMENTS
20.15 WILLKIE FARR & GALLAGHER LLP
20.15.1 COMPANY OVERVIEW
20.15.2 REVENUE ANALYSIS
20.15.3 GEOGRAPHIC PRESENCE
20.15.4 PRODUCT PORTFOLIO
20.15.5 RECENT DEVELOPMENTS
20.16 ASTRAZENECA
20.16.1 COMPANY OVERVIEW
20.16.2 REVENUE ANALYSIS
20.16.3 GEOGRAPHIC PRESENCE
20.16.4 PRODUCT PORTFOLIO
20.16.5 RECENT DEVELOPMENTS
20.17 PROTEON THERAPEUTICS, INC.
20.17.1 COMPANY OVERVIEW
20.17.2 REVENUE ANALYSIS
20.17.3 GEOGRAPHIC PRESENCE
20.17.4 PRODUCT PORTFOLIO
20.17.5 RECENT DEVELOPMENTS
20.18 APOTEX INC
20.18.1 COMPANY OVERVIEW
20.18.2 REVENUE ANALYSIS
20.18.3 GEOGRAPHIC PRESENCE
20.18.4 PRODUCT PORTFOLIO
20.18.5 RECENT DEVELOPMENTS
20.19 SYMIC BIO, INC.
20.19.1 COMPANY OVERVIEW
20.19.2 REVENUE ANALYSIS
20.19.3 GEOGRAPHIC PRESENCE
20.19.4 PRODUCT PORTFOLIO
20.19.5 RECENT DEVELOPMENTS
20.2 THERAVASC, INC.
20.20.1 COMPANY OVERVIEW
20.20.2 REVENUE ANALYSIS
20.20.3 GEOGRAPHIC PRESENCE
20.20.4 PRODUCT PORTFOLIO
20.20.5 RECENT DEVELOPMENTS
20.21 ENDOLOGIX LLC.
20.21.1 COMPANY OVERVIEW
20.21.2 REVENUE ANALYSIS
20.21.3 GEOGRAPHIC PRESENCE
20.21.4 PRODUCT PORTFOLIO
20.21.5 RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
21 RELATED REPORTS
22 CONCLUSION
23 QUESTIONNAIRE
24 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.