Global Pharmacological Chaperone Drug Market
Market Size in USD Million
CAGR :
% 
 USD
452.96 Million
USD
1,429.59 Million
2024
2032
USD
452.96 Million
USD
1,429.59 Million
2024
2032
| 2025 –2032 | |
| USD 452.96 Million | |
| USD 1,429.59 Million | |
|
|
|
|
Pharmacological Chaperone Drug Market Size
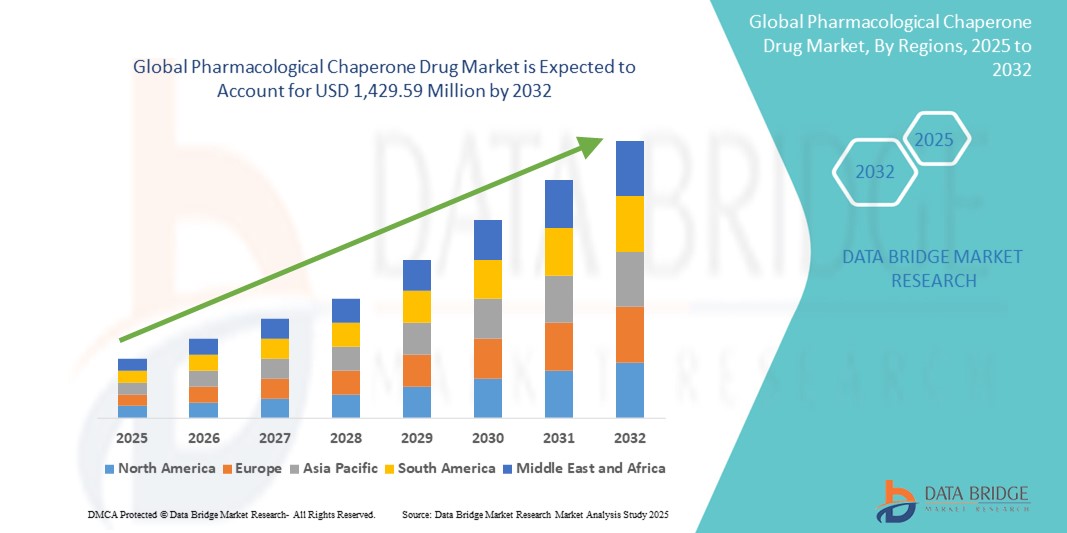
- The global pharmacological chaperone drug market size was valued at USD 452.96 million in 2024 and is expected to reach USD 1,429.59 million by 2032, at a CAGR of 15.45% during the forecast period
- The market growth is largely fueled by the increasing research and clinical interest in treating rare genetic disorders, particularly lysosomal storage diseases, through targeted small molecule therapies such as pharmacological chaperones. These drugs work by stabilizing misfolded proteins, enhancing their proper folding and functionality, and thereby addressing the root cause of several enzyme-deficiency conditions
- Furthermore, rising demand for precision medicine and the growing prevalence of rare and orphan diseases are positioning pharmacological chaperones as a promising therapeutic class. These converging factors are accelerating the adoption of pharmacological chaperone drug solutions, thereby significantly boosting the industry's growth
Pharmacological Chaperone Drug Market Analysis
- Pharmacological chaperones, which are small molecules that stabilize misfolded proteins and restore their normal function, are emerging as a vital therapeutic approach for treating genetic disorders, particularly lysosomal storage diseases and other protein-misfolding conditions. Their ability to selectively bind and stabilize target proteins has garnered increasing attention in the field of precision medicine
- The escalating demand for pharmacological chaperone therapies is primarily driven by a growing prevalence of rare genetic disorders, increased focus on targeted therapies, and advancements in drug discovery platforms such as structure-based drug design
- North America dominated the pharmacological chaperone drug market, accounting for the largest revenue share of 41.3% in 2024, due to early adoption of innovative therapeutics, robust R&D investments, supportive regulatory environment, and the strong presence of leading biopharmaceutical companies focused on rare disease treatment
- Asia-Pacific is projected to be the fastest-growing region in the pharmacological chaperone drug market during the forecast period of 2025 to 2032, with a CAGR of 13.8%, fueled by increasing awareness of rare genetic diseases, rising healthcare expenditure, growing biotechnology sector, and expanding access to diagnostics and advanced therapies in countries such as China, Japan, and India
- The Monotherapy segment held the largest market share of 58.9% in 2024, supported by the approval of stand-alone pharmacological chaperones such as Galafold for specific genotypes. These therapies offer targeted treatment options without the need for adjunctive therapies, simplifying treatment regimens and improving patient compliance, particularly in lysosomal storage disorders such as Fabry disease
Report Scope and Pharmacological Chaperone Drug Market Segmentation
|
Attributes |
Pharmacological Chaperone Drug Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Pharmacological Chaperone Drug Market Trends
“Growing Demand for Precision Therapeutics and Targeted Protein Stabilization”
- A significant and accelerating trend in the global pharmacological chaperone drug market is the rising demand for targeted therapies aimed at correcting protein misfolding associated with rare genetic and lysosomal storage disorders. Pharmacological chaperones work by stabilizing the structure of misfolded proteins, helping them reach their intended cellular destinations and restore partial or full function
- For instance, migalastat, a pharmacological chaperone approved for Fabry disease, exemplifies the therapeutic potential of these agents by selectively binding and stabilizing the mutant alpha-galactosidase A enzyme. This precise mechanism of action is fueling interest in expanding the application of chaperone drugs to other protein misfolding-related conditions
- The emergence of next-generation drug development platforms is enabling companies to identify and design small-molecule chaperones with improved selectivity, bioavailability, and safety profiles. These advancements are expanding the pipeline of investigational drugs, with several candidates in clinical trials for conditions such as Gaucher disease, Parkinson’s disease, and cystic fibrosis
- Moreover, the integration of companion diagnostics in pharmacological chaperone drug development is enhancing the ability to identify suitable patient populations, thereby improving clinical outcomes and supporting regulatory approvals
- This growing preference for precision medicine approaches is driving investments from both pharmaceutical companies and biotech firms, with key players increasingly forming strategic collaborations to accelerate research and development. For example, Amicus Therapeutics and GlaxoSmithKline have partnered to develop personalized chaperone-based therapies for lysosomal disorders
- The expanding role of pharmacological chaperones in the management of chronic and rare diseases is expected to significantly reshape treatment paradigms, providing new hope for patients with limited therapeutic options and fueling sustained market growth across global regions
Pharmacological Chaperone Drug Market Dynamics
Driver
“Growing Need Due to Rise in Protein Misfolding Disorders and Targeted Therapies”
- The increasing incidence of rare genetic disorders and protein misfolding diseases, such as Fabry disease, Gaucher disease, and certain forms of cystic fibrosis and Parkinson’s disease, is a major driver of the Pharmacological Chaperone Drug market. These conditions require precise, targeted interventions—which pharmacological chaperones offer—by stabilizing and correcting misfolded proteins at the molecular level
- For instance, in 2023, Amicus Therapeutics advanced its pharmacological chaperone candidate migalastat (Galafold) as a precision treatment for Fabry disease, showing significant clinical benefits in genetically defined patients. Such initiatives underscore the growing relevance of chaperone-based therapies and are expected to drive industry growth during the forecast period.
- Furthermore, rising awareness and increasing R&D investments in rare disease therapeutics are helping bring pharmacological chaperones into mainstream clinical practice. With advancements in genetic testing and diagnostics, patients can now be better stratified for personalized chaperone therapy
- The convenience of oral administration, improved pharmacokinetics, and the potential for long-term disease modification are propelling adoption across pharmaceutical pipelines and regulatory agencies. The trend towards orphan drug designation and fast-track approvals by regulatory bodies such as the FDA and EMA further contributes to market growth
- Moreover, the ability to combine pharmacological chaperones with enzyme replacement therapy (ERT) or gene therapy offers a versatile platform for managing complex diseases, appealing to researchers and biotech developers alike
Restraint/Challenge
“Limited Target Population and High Development Costs”
- A key challenge for the pharmacological chaperone drug market is its narrow patient population. Since most approved or in-development chaperones are intended for rare diseases, the limited number of eligible patients can affect the scalability and commercial viability of such therapies
- For instance, Galafold by Amicus Therapeutics is only indicated for a subset of Fabry patients with amenable mutations, narrowing its market reach
- Development of pharmacological chaperones also requires sophisticated molecular screening and high R&D investments, often with long clinical timelines. These factors can hinder broader market participation by smaller biotech firms
- In addition, regulatory hurdles—such as proving long-term efficacy, establishing biomarker-based endpoints, and ensuring precision in mutation-specific therapies—create barriers to approval and reimbursement
- Addressing these challenges will require public-private partnerships, funding for orphan drug programs, improved patient identification strategies, and continued innovation in protein stabilization science
Pharmacological Chaperone Drug Market Scope
The market is segmented on the basis of type, mechanism of action, therapy type, and application.
- By Type
On the basis of type, the pharmacological chaperone drug market is segmented into small molecule chaperones, protein-based chaperones, substrate reduction chaperones, and others. The small molecule chaperones segment held the largest revenue share of 46.7% in 2024, owing to their ability to bind selectively to misfolded proteins and facilitate proper folding, which is critical in treating rare genetic disorders such as Fabry and Gaucher diseases. These chaperones are often preferred due to their oral bioavailability and ease of administration.
The protein-based chaperones segment is expected to witness the fastest CAGR of 19.2% from 2025 to 2032, driven by advancements in protein engineering and increasing research on heat shock proteins (HSPs) and other naturally occurring chaperone systems to manage neurodegenerative conditions and lysosomal storage disorders.
- By Mechanism of Action
On the basis of mechanism of action, the pharmacological chaperone drug market is segmented into enzyme stabilization, protein refolding, receptor modulation, and others. Enzyme stabilization dominated the market in 2024 with a revenue share of 42.1%, attributed to the rising demand for therapies that enhance the stability and activity of defective enzymes, especially in lysosomal storage disorders. Drugs such as migalastat act via this mechanism.
Protein refolding is projected to be the fastest growing segment, with a CAGR of 17.6% from 2025 to 2032, as research accelerates into correcting misfolded proteins in diseases such as Parkinson’s and cystic fibrosis, where restoring native conformation offers disease-modifying potential.
- By Therapy Type
On the basis of therapy type, the pharmacological chaperone drug market is segmented into monotherapy and combination therapy. Monotherapy held the largest market share of 58.9% in 2024, supported by the approval of stand-alone pharmacological chaperones such as Galafold for specific genotypes.
Combination Therapy is expected to grow at a fastest CAGR of 18.3% from 2025 to 2032, as chaperones are increasingly being co-administered with enzyme replacement therapy (ERT) or substrate reduction therapies to improve efficacy and extend therapeutic reach.
- By Application
On the basis of application, the pharmacological chaperone drug market is segmented into lysosomal storage disorders, parkinson’s disease, cystic fibrosis, epilepsy, and others. Lysosomal storage disorders accounted for the largest revenue share of 51.6% in 2024, driven by high unmet medical needs and favorable regulatory pathways such as orphan drug designations and fast-track approvals.
Parkinson’s Disease is anticipated to grow at the highest CAGR of 20.5% from 2025 to 2032, fueled by research investments into misfolded alpha-synuclein and ongoing clinical trials targeting neurodegenerative proteinopathies with precision chaperones.
Pharmacological Chaperone Drug Market Regional Analysis
- North America dominated the pharmacological chaperone drug market with the largest revenue share of 41.3% in 2024, driven by a robust pipeline of therapies for rare diseases, increasing prevalence of lysosomal storage disorders, and supportive regulatory pathways for orphan drugs. A strong presence of biopharmaceutical companies, growing R&D investments, and collaborations between academic institutions and industry further propel the regional market
- The growing demand for personalized and precision medicine, combined with favorable reimbursement policies and fast-track drug approval programs in the region, is fostering rapid innovation in pharmacological chaperone drug development
- This momentum is reinforced by high healthcare expenditure, increasing awareness of rare and genetic diseases, and strategic alliances for clinical trials and commercialization
U.S. Pharmacological Chaperone Drug Market Insight
The U.S. pharmacological chaperone drug market captured the largest revenue share of 81% in 2024 within North America, fueled by extensive research capabilities, rising incidence of genetic and neurodegenerative diseases, and initiatives supporting orphan drug development. The U.S. FDA’s supportive regulatory framework, including orphan drug designation and breakthrough therapy designations, has attracted significant investments from biotech firms. Additionally, the presence of major players such as Amicus Therapeutics and Protalix BioTherapeutics is driving therapeutic advancements in Fabry disease, Parkinson’s disease, and Gaucher disease.
Europe Pharmacological Chaperone Drug Market Insight
The Europe pharmacological chaperone drug market is projected to grow at a significant CAGR over the forecast period, driven by growing awareness of rare diseases and government-backed initiatives to support early diagnosis and treatment. The region’s strong biomedical research ecosystem and support from regulatory bodies such as the EMA are encouraging the development and market access of pharmacological chaperones. Rising patient advocacy, clinical trial activity, and collaborations across the EU further enhance the market’s growth potential.
U.K. Pharmacological Chaperone Drug Market Insight
The U.K. pharmacological chaperone drug market is expected to expand steadily due to increasing R&D in the domain of neurodegenerative and metabolic disorders, government funding for rare diseases, and growing collaborations between pharmaceutical companies and research universities. The U.K.’s focus on genomic medicine and its National Rare Diseases Framework are fostering early-stage innovation and clinical access to personalized therapies, including pharmacological chaperones.
Germany Pharmacological Chaperone Drug Market Insight
The Germany pharmacological chaperone drug market is anticipated to register consistent growth, supported by a well-developed biopharmaceutical sector, strong intellectual property protections, and a focus on advanced therapeutics for complex conditions. Germany’s proactive approach toward rare disease management, combined with patient-centric initiatives and hospital-based research networks, is driving the adoption of pharmacological chaperone therapies across multiple indications.
Asia-Pacific Pharmacological Chaperone Drug Market Insight
The Asia-Pacific pharmacological chaperone drug market is expected to grow at the fastest CAGR of 13.8% during 2025–2032, owing to a surge in rare disease diagnoses, improving healthcare infrastructure, and expanding biotech capabilities in countries such as China, Japan, and India. Government support through orphan drug frameworks, increasing clinical trials, and growing awareness among clinicians and patients are collectively boosting market penetration in this region.
Japan Pharmacological Chaperone Drug Market Insight
The Japan pharmacological chaperone drug market is gaining traction due to its advanced biotechnology ecosystem, government incentives for rare disease drug development, and rising demand for novel therapeutics addressing unmet medical needs. The Pharmaceutical and Medical Device Agency (PMDA) facilitates expedited approvals for orphan drugs, while Japan’s aging population and rising burden of Parkinson’s and lysosomal storage disorders support increased market adoption.
China Pharmacological Chaperone Drug Market Insight
The China pharmacological chaperone drug market held the largest revenue share in Asia-Pacific in 2024, driven by a growing middle class, rapid expansion in rare disease diagnostics, and strategic focus on healthcare innovation. National policies such as the First Rare Disease List and improved access to genetic testing are enhancing disease awareness and diagnosis. Additionally, the presence of local biotech firms and favorable clinical trial reforms are enabling accelerated drug development and commercialization of pharmacological chaperone therapies.
Pharmacological Chaperone Drug Market Share
The pharmacological chaperone drug industry is primarily led by well-established companies, including:
- Amicus Therapeutics (U.S.)
- Pfizer Inc. (U.S.)
- Sanofi S.A. (France)
- Takeda Pharmaceutical Company Limited (Japan)
- BioMarin Pharmaceutical Inc. (U.S.)
- Sumitomo Pharma Co., Ltd. (Japan)
- Protalix BioTherapeutics, Inc. (Israel)
- Novartis AG (Switzerland)
- GSK plc (U.K.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- AstraZeneca (U.K.)
- Merck & Co., Inc. (U.S.)
- Theranexus (France)
- Chiesi Farmaceutici S.p.A. (Italy)
- Orphazyme A/S (Denmark)
- Genzyme Corporation (U.S.)
- Zymenex A/S (Denmark)
- Greenovation Biotech GmbH (Germany)
- Actelion Pharmaceuticals (Switzerland)
Latest Developments in Global Pharmacological Chaperone Drug Market
- In December 2023, Amicus Therapeutics announced that its pharmacological chaperone drug Galafold (migalastat) continues to expand its global footprint, with approvals in over 40 countries for treating Fabry disease in patients with amenable GLA variants. The therapy has now been adopted by over 2,400 patients globally. Galafold remains the first and only oral monotherapy approved in multiple markets for Fabry disease
- In January 2024, Teva Pharmaceutical Industries Ltd. entered into a licensing agreement with Amicus Therapeutics to commercialize a generic version of Galafold in the United States. The agreement permits Teva to launch the product no earlier than January 2037, ensuring future market competition while preserving access
- In September 2023, Georgia Tech researchers received a grant from BrightFocus Foundation to develop a novel pharmacological chaperone targeting mutant myocilin protein, a key factor in inherited primary open-angle glaucoma. The aim is to stabilize the protein structure and prevent disease progression, a potential breakthrough in ophthalmic chaperone therapies
- In October 2023, a peer-reviewed study published in Pharmaceuticals (MDPI) showcased the growing potential of non-inhibitory pharmacological chaperones, which stabilize misfolded proteins without inhibiting enzyme function. This class is being explored for diseases including Gaucher, Pompe, and Tay–Sachs
- In November 2023, Ambroxol—a known mucolytic agent—was highlighted in clinical trials for its pharmacological chaperone properties in Gaucher disease type 3, demonstrating ability to enhance glucocerebrosidase function in neuronal cells, a promising approach for neuronopathic forms of the disease
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.