Global Phelan Mcdermid Syndrome Market
Market Size in USD Billion
CAGR :
% 
 USD
1.70 Billion
USD
2.45 Billion
2024
2032
USD
1.70 Billion
USD
2.45 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 2.45 Billion | |
|
|
|
|
Phelan McDermid Syndrome Market Size
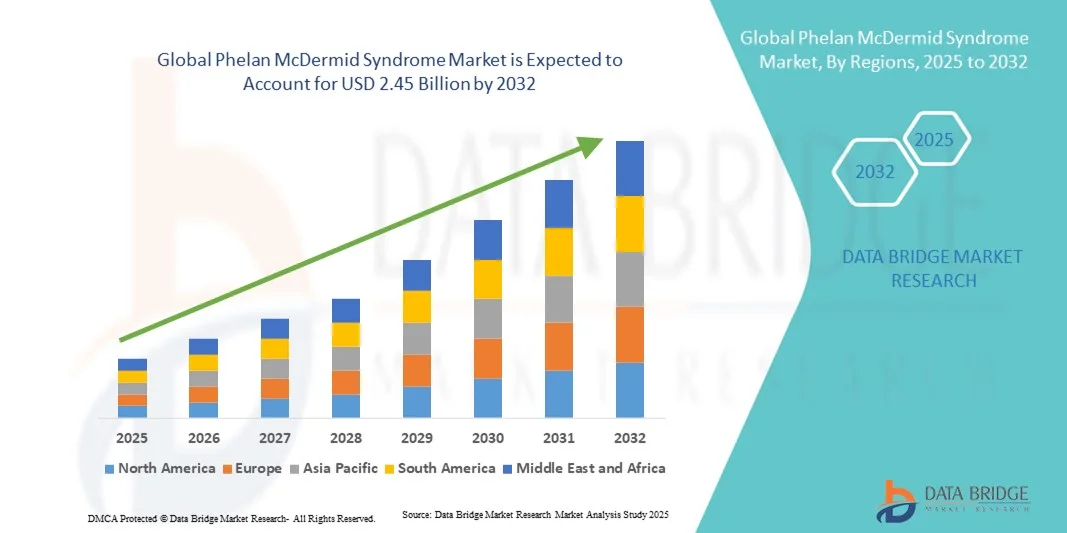
- The global Phelan McDermid Syndrome market size was valued at USD 1.70 billion in 2024 and is expected to reach USD 2.45 billion by 2032, at a CAGR of 4.70% during the forecast period
- The market growth is primarily driven by increasing research initiatives, rising awareness among healthcare professionals, and the growing number of genetic testing programs aiding in early diagnosis of rare neurodevelopmental disorders
- In addition, the expanding pipeline of targeted therapies and gene-based research aimed at addressing SHANK3 gene deletions is enhancing the treatment landscape. These developments, coupled with collaborations among research institutions and biotech firms, are accelerating innovation and shaping a more robust market outlook for Phelan-McDermid Syndrome globally
Phelan McDermid Syndrome Market Analysis
- Phelan-McDermid Syndrome (PMS), a rare genetic disorder caused by deletions or mutations in the SHANK3 gene, is gaining substantial clinical and research interest due to its association with autism spectrum disorder and intellectual disabilities, driving the demand for advanced diagnostics and targeted treatments
- The market growth is primarily driven by technological advancements in genetic sequencing, increased awareness among healthcare professionals, and expanding funding for rare disease research by both government and private sectors
- North America dominated the Phelan-McDermid Syndrome market with the largest revenue share of 43.9% in 2024, supported by strong research infrastructure, well-established diagnostic networks, and the active involvement of genetic testing laboratories and patient advocacy organizations
- Asia-Pacific is projected to be the fastest-growing region during the forecast period, driven by growing accessibility to genetic testing, increasing healthcare expenditure, and greater focus on early detection of rare genetic disorders
- The Chromosomal Microarray Analysis (CMA) segment dominated the market with a market share of 47.2% in 2024, owing to its proven efficiency in identifying chromosomal deletions and duplications, making it the preferred diagnostic method in hospitals and specialty clinics for confirming SHANK3-related abnormalities
Report Scope and Phelan McDermid Syndrome Market Segmentation
|
Attributes |
Phelan McDermid Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Phelan McDermid Syndrome Market Trends
Advancements in Genetic and Molecular Research for Targeted Therapies
- A significant and accelerating trend in the global Phelan-McDermid Syndrome (PMS) market is the growing focus on genetic and molecular research aimed at developing targeted and personalized therapies that address the underlying SHANK3 gene mutations responsible for the disorder
- For instance, in February 2024, the Phelan-McDermid Syndrome Foundation collaborated with the Seaver Autism Center for Research and Treatment to expand genomic research efforts, aiming to identify potential therapeutic targets and biomarkers
- The use of advanced genomic tools such as CRISPR-based editing, RNA modulation, and antisense oligonucleotide therapies is enabling researchers to explore precision treatment approaches for PMS, potentially offering long-term disease management solutions
- Furthermore, integration of AI-driven genomic analytics is helping in mapping complex gene interactions, improving diagnosis accuracy, and optimizing candidate drug discovery pipelines for SHANK3-related disorders
- The expansion of global biobank initiatives and rare disease registries is fostering large-scale data sharing, accelerating clinical trials, and enabling more accurate phenotype-genotype correlations for PMS patients worldwide
- This growing emphasis on molecular-level innovation is transforming the therapeutic landscape, encouraging biotech firms and research institutions to invest in next-generation genetic treatment modalities for rare neurodevelopmental disorders such as PMS
Phelan McDermid Syndrome Market Dynamics
Driver
Rising Diagnostic Awareness and Research Collaborations in Rare Diseases
- The increasing awareness of rare genetic disorders, combined with growing global initiatives to improve diagnostic capabilities and patient outcomes, is a key driver for the Phelan-McDermid Syndrome market
- For instance, in June 2024, the U.S. National Institutes of Health (NIH) expanded funding for rare disease research, supporting multi-institutional collaborations focused on understanding SHANK3 gene-related pathologies
- As healthcare systems emphasize early genetic screening and precision medicine, the adoption of diagnostic technologies such as Chromosomal Microarray Analysis (CMA) and Whole Exome Sequencing (WES) has significantly improved PMS detection rates
- Furthermore, the involvement of patient advocacy organizations and rare disease networks is accelerating information dissemination, leading to faster diagnosis and clinical trial participation for affected families
- The increasing availability of research grants, along with partnerships between academia and biotechnology companies, is catalyzing the development of innovative therapeutics specifically targeting SHANK3 mutations and related neurological symptoms
- The rapid advancement of genomic infrastructure in both developed and emerging regions is supporting the early detection, treatment development, and long-term management of PMS, driving market expansion globally
Restraint/Challenge
Limited Treatment Options and High Cost of Genetic Testing
- The lack of approved targeted therapies and limited understanding of the complex molecular mechanisms of Phelan-McDermid Syndrome remain significant challenges restricting broader market growth
- For instance, despite ongoing research, no curative treatment currently exists, and available management options remain largely supportive, focusing on symptom relief rather than underlying gene correction
- The high cost of advanced genetic testing technologies, including Whole Exome Sequencing (WES) and specialized chromosomal analysis, poses a financial burden for patients, particularly in low- and middle-income countries
- Furthermore, the rarity of PMS often leads to delayed diagnosis, limited clinical trial enrollment, and a lack of standardized treatment protocols across global healthcare systems
- Reimbursement challenges and the absence of dedicated funding programs for ultra-rare disorders can further hinder patient access to testing and emerging therapies, limiting market penetration
- Overcoming these barriers through international research collaborations, improved healthcare funding, and expansion of affordable diagnostic infrastructure will be crucial for unlocking the full growth potential of the PMS market
Phelan McDermid Syndrome Market Scope
The market is segmented on the basis of drugs & diagnostic technologies, diagnosis, and end-users.
- By Drugs & Diagnostic Technologies
On the basis of drugs and diagnostic technologies, the Phelan-McDermid Syndrome market is segmented into chromatin structures, micro RNA modification, large non-coding rna, histone acetylation, histone methylation, and DNA methylation. The DNA Methylation segment dominated the market with the largest revenue share in 2024, driven by its central role in regulating gene expression and its relevance to SHANK3-related neurodevelopmental abnormalities. Researchers increasingly rely on DNA methylation profiling to understand gene silencing mechanisms contributing to PMS pathology. This method provides critical insights into epigenetic regulation, guiding therapeutic development and diagnostic innovation. The widespread adoption of DNA methylation analysis in genetic and neurological research has strengthened its position as a leading diagnostic technology for rare genetic disorders, including PMS. Moreover, its growing use in biomarker discovery and therapeutic response monitoring further supports its dominance in the market.
The Micro RNA Modification segment is anticipated to witness the fastest growth rate during the forecast period (2025–2032), propelled by emerging studies demonstrating the regulatory role of microRNAs in neuronal development and SHANK3 expression. Advances in molecular biology have enabled researchers to target specific microRNAs involved in PMS pathophysiology, potentially opening new therapeutic avenues. For instance, ongoing research efforts focus on modulating microRNA expression to restore neuronal signaling and synaptic balance. The increasing adoption of RNA-based diagnostic platforms and gene-silencing technologies is further accelerating this segment’s expansion.
- By Diagnosis
On the basis of diagnosis, the market is segmented into chromosomal microarray analysis (CMA), single-gene testing, and whole exome sequencing (WES). The Chromosomal Microarray Analysis (CMA) segment dominated the market with the largest revenue share of 47.2% in 2024, owing to its high accuracy and efficiency in detecting deletions or duplications in chromosome 22q13, where the SHANK3 gene is located. CMA is considered the gold-standard diagnostic tool for identifying Phelan-McDermid Syndrome due to its reliability in uncovering copy number variations linked to the disorder. The growing integration of CMA in clinical genetic testing protocols and its cost-effectiveness compared to full exome sequencing contribute to its leadership. Hospitals and diagnostic centers prefer CMA because it offers rapid results and is widely reimbursed by healthcare systems for rare disease detection.
The Whole Exome Sequencing (WES) segment is projected to witness the fastest growth from 2025 to 2032, driven by its comprehensive ability to detect a wide range of genetic mutations, including single nucleotide variants and small insertions/deletions. WES is increasingly used when CMA or single-gene testing fails to identify causative mutations, making it a valuable second-tier diagnostic tool. The declining cost of next-generation sequencing (NGS) technologies and their ability to provide a holistic view of the genome are key growth accelerators. Furthermore, WES supports precision medicine initiatives and research programs aimed at uncovering new gene-disease correlations in PMS and related neurological disorders.
- By End-Users
On the basis of end-users, the Phelan-McDermid Syndrome market is segmented into hospitals, homecare, and specialty clinics. The Hospitals segment dominated the market with the largest revenue share in 2024, driven by the high concentration of advanced diagnostic facilities, multidisciplinary medical expertise, and access to genetic counseling services. Hospitals play a pivotal role in PMS diagnosis, patient management, and participation in clinical trials for emerging therapies. They also serve as the primary centers for genetic testing, molecular research, and patient registries. The increasing collaboration between hospital-based research units and biotech firms developing SHANK3-targeted therapeutics further strengthens this segment’s dominance. In addition, government-funded rare disease programs often channel research and patient care through hospital systems, reinforcing their market leadership.
The Specialty Clinics segment is anticipated to grow at the fastest CAGR from 2025 to 2032, owing to the rising establishment of rare disease and neurodevelopmental disorder clinics offering personalized genetic counseling and long-term management solutions. These clinics cater specifically to patients requiring individualized care, rehabilitation, and monitoring, particularly for neurobehavioral and cognitive symptoms of PMS. The expansion of specialty genetics and neurology clinics in both developed and emerging markets is driving access to expert-led diagnostics. Moreover, the growing integration of telemedicine and remote monitoring services within specialty clinics enhances their ability to provide continuous support and follow-up for PMS patients, fueling segment growth.
Phelan McDermid Syndrome Market Regional Analysis
- North America dominated the Phelan-McDermid Syndrome market with the largest revenue share of 43.9% in 2024, supported by strong research infrastructure, well-established diagnostic networks, and the active involvement of genetic testing laboratories and patient advocacy organization
- Patients and healthcare providers in the region increasingly prioritize early diagnosis through advanced genetic testing methods such as Chromosomal Microarray Analysis (CMA) and Whole Exome Sequencing (WES), supported by favorable reimbursement policies and government-backed rare disease programs
- This leadership is further reinforced by active collaborations between hospitals, universities, and rare disease foundations, along with rising participation in clinical trials targeting SHANK3 gene therapies, positioning North America as the central hub for innovation and patient care in the global Phelan-McDermid Syndrome market
U.S. Phelan McDermid Syndrome Market Insight
The U.S. Phelan-McDermid Syndrome market captured the largest revenue share of 82% in 2024 within North America, driven by extensive rare disease research programs and strong government support for genetic disorder initiatives. The growing adoption of advanced diagnostic tools such as Chromosomal Microarray Analysis (CMA) and Whole Exome Sequencing (WES) is enhancing early detection rates. Increased funding from organizations such as the NIH and active participation in clinical trials targeting SHANK3 gene therapies further propel market growth. Moreover, the presence of major biotechnology companies and specialized research institutions fosters innovation, positioning the U.S. as a leader in PMS diagnosis and treatment development.
Europe Phelan-McDermid Syndrome Market Insight
The Europe Phelan-McDermid Syndrome market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing awareness of rare genetic disorders and strong collaboration among research networks. Supportive healthcare policies and government funding for orphan disease research are facilitating early diagnosis and patient care. European healthcare systems are emphasizing genetic counseling and molecular diagnostics integration in clinical workflows. The region is witnessing significant research participation across countries such as Germany, France, and the U.K., focusing on gene-targeted therapies and long-term patient registries.
U.K. Phelan-McDermid Syndrome Market Insight
The U.K. Phelan-McDermid Syndrome market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by a growing focus on rare disease awareness, genomic research, and national healthcare initiatives. Programs such as Genomics England are accelerating genetic testing accessibility, supporting earlier identification of SHANK3 deletions. Increasing collaboration between academic institutions and pharmaceutical companies is fostering advancements in gene-based research. Furthermore, strong patient advocacy organizations and national registries are improving data collection and clinical coordination, enhancing care outcomes for PMS patients across the country.
Germany Phelan-McDermid Syndrome Market Insight
The Germany Phelan-McDermid Syndrome market is expected to expand at a considerable CAGR during the forecast period, supported by robust investment in biomedical research and a well-established healthcare infrastructure. Germany’s strong focus on translational genetics and molecular diagnostics is enabling progress in PMS detection and therapeutic development. Hospitals and research centers actively participate in EU-funded projects to explore SHANK3-related pathways. In addition, the emphasis on digital health innovation and sustainable healthcare models is enhancing access to genetic testing and specialized care, promoting early and accurate diagnosis across the nation.
Asia-Pacific Phelan-McDermid Syndrome Market Insight
The Asia-Pacific Phelan-McDermid Syndrome market is poised to grow at the fastest CAGR of 9.2% during the forecast period (2025–2032), driven by expanding access to genetic testing and increasing awareness of neurodevelopmental disorders in countries such as China, Japan, and India. Rapid healthcare digitalization and government initiatives for rare disease management are promoting diagnostic advancements. Regional growth is also supported by collaborations between local hospitals and international research organizations. As healthcare infrastructure strengthens and awareness campaigns expand, more patients are being identified and referred for early intervention and genetic counseling.
Japan Phelan-McDermid Syndrome Market Insight
The Japan Phelan-McDermid Syndrome market is gaining momentum due to the country’s strong biotechnology sector, advanced genomic research programs, and focus on neurogenetic disorders. Japan’s integration of precision medicine and next-generation sequencing technologies supports accurate detection and clinical study participation. For instance, academic hospitals and research institutions are leading studies on SHANK3 gene mutations to develop personalized treatment options. The country’s emphasis on technological innovation and a coordinated healthcare approach for rare diseases further enhances market expansion.
India Phelan-McDermid Syndrome Market Insight
The India Phelan-McDermid Syndrome market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the rapid adoption of genetic testing technologies, rising healthcare investments, and government-driven rare disease programs. India’s expanding network of diagnostic laboratories and increasing participation in global research collaborations are improving disease identification and awareness. The growing middle-class population and improved access to healthcare are supporting patient screening initiatives. Moreover, domestic biotechnology startups focusing on gene diagnostics and sequencing are accelerating accessibility and affordability of PMS testing across the nation.
Phelan McDermid Syndrome Market Share
The Phelan McDermid Syndrome industry is primarily led by well-established companies, including:
- Jaguar Gene Therapy, LLC (U.S.)
- NeuroNOS (U.S.)
- Neuren Pharmaceuticals (Australia)
- CureSHANK (U.S.)
- Phelan-McDermid Syndrome Foundation (U.S.)
- Illumina, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- QIAGEN (Netherlands)
- BGI Group (China)
- Labcorp (U.S.)
- Fulgent Genetics (U.S.)
- Sema4.ai (U.S.)
- Bionano Genomics, Inc. (U.S.)
- PerkinElmer (U.S.)
- Agilent Technologies, Inc. (U.S.)
- Oxford Nanopore Technologies plc (U.K.)
- Adaptive Biotechnologies (U.S.)
- Taysha GTx (U.S.)
- Passage Bio (U.S.)
What are the Recent Developments in Global Phelan McDermid Syndrome Market?
- In April 2025, NeuroNOS, a neuroscience-focused biopharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation (ODD) to its investigational therapy BA-102 for the treatment of Phelan-McDermid Syndrome (PMS). The therapy targets SHANK3-haploinsufficiency, a key genetic cause of PMS, using a novel neuroprotective mechanism to restore synaptic function
- In March 2025, Jaguar Gene Therapy officially announced the initiation of patient enrollment for its first-in-human clinical trial of JAG201, a gene therapy candidate designed to treat Phelan-McDermid Syndrome and SHANK3-related autism. The company confirmed that the clinical study had opened for screening and enrollment following successful preclinical safety and efficacy data
- In October 2024, the Phelan-McDermid Syndrome Foundation (PMSF) announced the allocation of three new research grants aimed at improving diagnosis and therapeutic evaluation in PMS. Among the funded initiatives was the development of PIPS for Progress, a digital behavioral assessment platform designed to track real-time changes in social interaction, cognition, and motor function in PMS patient
- In July 2024, Jaguar Gene Therapy announced that it had received positive feedback from the FDA regarding its Type C meeting for the JAG201 program targeting Phelan-McDermid Syndrome. The regulatory response allowed the company to move forward with a Phase I clinical trial, marking the first FDA-authorized gene therapy trial focused on the SHANK3 gene deficiency
- In April 2024, CureSHANK, a nonprofit organization dedicated to accelerating SHANK3-related research, hosted the inaugural Phelan-McDermid Syndrome Drug Development Symposium in Boston, Massachusetts. The event brought together leading researchers, biotechnology executives, patient advocacy leaders, and regulatory experts to foster collaboration across academia and industry
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.