Global Phenylpropanolamine Ppa Market
Market Size in USD Billion
CAGR :
% 
 USD
1.22 Billion
USD
1.92 Billion
2024
2032
USD
1.22 Billion
USD
1.92 Billion
2024
2032
| 2025 –2032 | |
| USD 1.22 Billion | |
| USD 1.92 Billion | |
|
|
|
|
Phenylpropanolamine (PPA) Market Size
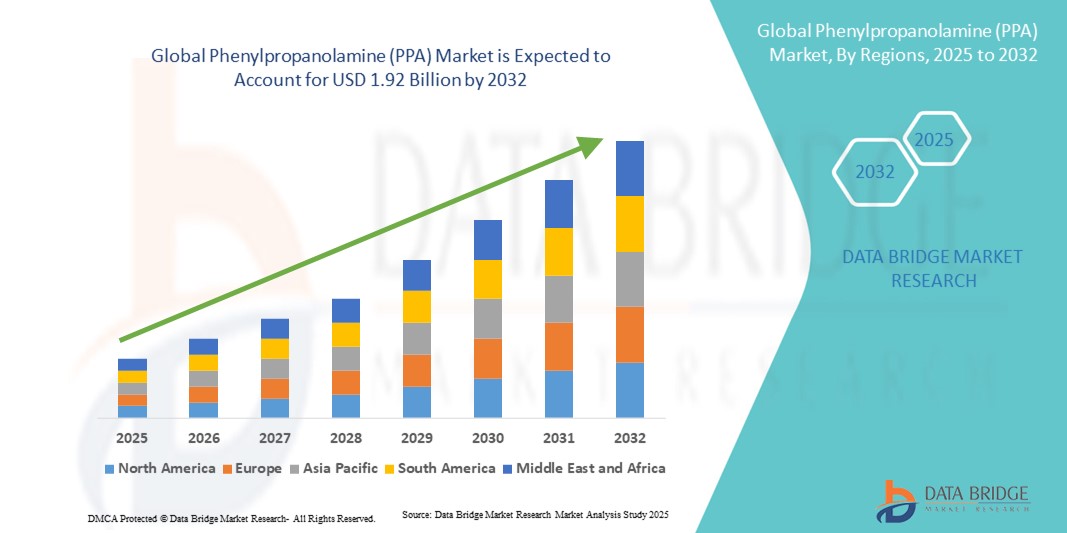
- The global phenylpropanolamine (PPA) market size was valued at USD 1.22 billion in 2024 and is expected to reach USD 1.92 billion by 2032, at a CAGR of 5.8% during the forecast period
- The market growth is largely fueled by the rising demand for PPA in pharmaceutical formulations, particularly as a nasal decongestant and appetite suppressant, alongside its applications in veterinary medicine. Increasing healthcare expenditure and the expansion of the generic drug industry are further contributing to its adoption across emerging markets
- Furthermore, regulatory reevaluations, advancements in safer formulations, and the growing demand for effective cold, flu, and weight management treatments are shaping the industry landscape. These converging factors are sustaining the uptake of PPA-based products, thereby supporting steady market expansion
Phenylpropanolamine (PPA) Market Analysis
- Phenylpropanolamine (PPA), a sympathomimetic agent widely used as a nasal decongestant, appetite suppressant, and veterinary therapeutic, remains a key active pharmaceutical ingredient (API) in respiratory and cold-related formulations due to its proven efficacy and affordability across diverse healthcare markets
- The escalating demand for PPA is primarily fueled by the rising prevalence of colds, flu, and respiratory allergies, growing pharmaceutical production in emerging economies, and increasing reliance on cost-effective over-the-counter (OTC) formulations for symptomatic relief
- North America dominated the phenylpropanolamine market with the largest revenue share of 38.1% in 2024, driven by advanced pharmaceutical manufacturing capabilities, established healthcare infrastructure, and consistent usage in both prescription and veterinary drugs, with the U.S. leading due to strong demand for cold and flu medications
- Asia-Pacific is expected to be the fastest growing region in the phenylpropanolamine market during the forecast period, fueled by population growth, rising healthcare spending, and the rapid expansion of pharmaceutical manufacturing hubs in China and India
- The human application segment dominated the phenylpropanolamine market with a market share of 71.3% in 2024, owing to its extensive use in cold, flu, and allergy treatments, coupled with increasing demand for accessible and effective formulations across adult populations
Report Scope and Phenylpropanolamine (PPA) Market Segmentation
|
Attributes |
Phenylpropanolamine (PPA) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Phenylpropanolamine (PPA) Market Trends
Rising Demand for Affordable Cold and Allergy Treatments
- A significant and accelerating trend in the global phenylpropanolamine (PPA) market is the growing demand for cost-effective treatments for colds, hay fever, sinusitis, and respiratory allergies, especially in emerging economies. Increasing reliance on over-the-counter (OTC) medications and generic formulations is expanding PPA usage
- For instance, several regional pharmaceutical companies in Asia and Latin America are strengthening their cold and flu product portfolios with PPA-based formulations due to high patient affordability and accessibility
- The development of combination therapies, where PPA is paired with antihistamines or analgesics, is further broadening its applications by offering multi-symptom relief in a single dosage
- In addition, PPA continues to play a vital role in veterinary medicine, with increasing demand for effective and economical solutions for pet and livestock respiratory conditions
- The growing acceptance of solid dosage forms, coupled with advancements in safer reformulations to address regulatory concerns, is reinforcing PPA’s role in global pharmaceutical markets
- This trend toward affordable, multi-use, and reformulated PPA-based drugs is shaping a resilient market trajectory, particularly in regions where healthcare cost sensitivity remains high
Phenylpropanolamine (PPA) Market Dynamics
Driver
Rising Prevalence of Respiratory Ailments and OTC Drug Usage
- The increasing global incidence of respiratory ailments, such as colds, sinusitis, and allergies, is a significant driver for the demand for PPA-based treatments
- For instance, in 2024, several generic pharmaceutical manufacturers in India and China announced expanded production of PPA-containing medications to meet rising seasonal demand during peak cold and flu periods
- Growing preference for OTC solutions among adults, coupled with the availability of low-cost formulations in retail and online pharmacies, is accelerating market adoption
- Furthermore, the expansion of pharmaceutical manufacturing capabilities in Asia-Pacific and Latin America is strengthening the availability and affordability of PPA medicines across both developed and emerging markets
- Widespread use in veterinary applications and the convenience of established formulations further contribute to the steady demand for PPA across healthcare systems worldwide
Restraint/Challenge
Regulatory Restrictions and Safety Concerns
- The regulatory scrutiny surrounding the safety of phenylpropanolamine, particularly related to risks of adverse cardiovascular and neurological effects, poses a major challenge to market growth. In several developed markets, restrictions or controlled usage have limited its adoption in certain indications
- For instance, past safety evaluations by U.S. FDA and European authorities have led to tightened regulations on PPA in human medicines, prompting cautious consumer and physician adoption
- Pharmaceutical companies must navigate these compliance hurdles through reformulation strategies, safety trials, and transparent labeling to build confidence among regulators and consumers
- In addition, increasing awareness of potential side effects may reduce preference for PPA-based products in favor of alternatives, particularly in markets with strong regulatory enforcement
- Overcoming these challenges will require investment in safer PPA formulations, stronger pharmacovigilance, and market education efforts to sustain its role as an affordable and effective treatment option
Phenylpropanolamine (PPA) Market Scope
The market is segmented on the basis of indication, chemistry, application, drug type, population type, dosage form, end user, and distribution channel.
- By Indication
On the basis of indication, the phenylpropanolamine (PPA) market is segmented into colds, hay fever, upper respiratory allergies, sinusitis, and others. Colds segment dominated the market with the largest share of 36.8% in 2024, as PPA is widely used in over-the-counter (OTC) and prescription formulations to alleviate nasal congestion and other cold symptoms. Its affordability, established efficacy, and widespread availability make it a preferred choice among adults and children. Seasonal recurrence of colds, combined with global consumer awareness of quick relief treatments, ensures consistent demand. Pharmaceutical companies continue to strengthen their cold medicine portfolios, further supporting this segment. The ease of administration in tablets and liquid syrups reinforces its dominance.
Upper Respiratory Allergies is expected to witness the fastest growth at a CAGR of 8.6% from 2025 to 2032, driven by rising prevalence of allergic rhinitis, sinusitis, and other respiratory conditions. Urban pollution, climate change, and higher exposure to allergens have intensified demand for PPA-based allergy treatments. Emerging economies are increasingly adopting OTC solutions, further fueling growth. Pharmaceutical R&D efforts are focusing on combination therapies, where PPA is paired with antihistamines for multi-symptom relief. Growing awareness of affordable and effective allergy medications also supports adoption.
- By Chemistry
On the basis of chemistry, the phenylpropanolamine (PPA) market is segmented into D- and L-Norephedrine and D- and L-Norpseudoephedrine. D- and L-Norephedrine dominated with a share of 42.5% in 2024, due to its extensive use in nasal decongestants and appetite suppressant formulations. Its proven efficacy and regulatory acceptance in multiple countries ensure consistent demand. The wide application in both OTC and prescription medications strengthens its position. Pharmaceutical manufacturers rely on this chemistry for stable, cost-effective formulations. It is favored in solid dosage forms, which dominate global PPA distribution. Consumer familiarity and trust further contribute to its leading position.
D- and L-Norpseudoephedrine is projected to be the fastest-growing subsegment with a CAGR of 7.9% from 2025 to 2032, primarily due to its increasing use in weight management and appetite suppressant products. Rising obesity rates worldwide have created new market opportunities for effective pharmaceutical interventions. Pharmaceutical companies are investing in controlled formulations to meet safety standards. The segment also benefits from regulatory approvals in emerging markets. Rising adult population seeking weight control solutions supports this growth. Combination products containing this chemistry are also gaining traction in OTC markets.
- By Application
On the basis of application, the phenylpropanolamine (PPA) market is segmented into human and veterinary. Human application dominated the market with a share of 71.3% in 2024, as PPA is extensively used in cold, flu, and allergy treatments. Its inclusion in OTC medications ensures wide accessibility. Adult populations represent the primary users due to high prevalence of respiratory ailments. Pharmaceutical companies continue to strengthen human-centric formulations. The segment benefits from seasonal demand spikes during peak cold and flu periods. Solid dosage forms further reinforce adoption in human healthcare.
Veterinary application is expected to grow the fastest at a CAGR of 7.4% during 2025–2032, driven by increasing pet ownership and demand for livestock healthcare solutions. PPA is used for urinary incontinence and respiratory treatments in animals. Veterinary healthcare expansion in emerging economies is boosting market penetration. Pet owners and livestock managers increasingly prefer cost-effective medications. Specialty veterinary formulations further enhance segment growth. Growing awareness of animal health contributes to adoption.
- By Drug Type
On the basis of drug type, the phenylpropanolamine (PPA) market is segmented into Over-the-Counter (OTC) and prescription. Over-the-Counter (OTC) drugs dominated with a share of 58.6% in 2024, as consumers prefer convenient access to cold, flu, and allergy medications. Retail pharmacies and online platforms ensure widespread availability. OTC PPA formulations are cost-effective, appealing to budget-conscious users. Seasonal spikes in cold prevalence drive recurring demand. Consumers increasingly favor OTC solutions for mild conditions. The segment benefits from established trust and brand recognition.
Prescription drugs are expected to grow the fastest at a CAGR of 7.2% from 2025 to 2032, as physicians prescribe PPA in controlled doses for chronic allergic conditions and weight management therapies. Regulatory compliance in developed markets supports safe usage. Prescription formulations often include advanced delivery mechanisms for targeted efficacy. Emerging markets are witnessing growing prescription adoption. Physician awareness and patient education further encourage usage. Controlled dosing ensures patient safety and expands market potential.
- By Population Type
On the basis of population type, the phenylpropanolamine (PPA) market is segmented into children and adults. Adults dominated with a share of 67.4% in 2024, due to higher incidence of colds, allergies, and obesity-related conditions. Adults represent the largest consumer base for both OTC and prescription PPA products. Seasonal spikes in adult respiratory ailments reinforce consistent demand. Pharmaceutical manufacturers focus primarily on adult formulations. Easy-to-administer solid tablets further increase adoption. Adult self-medication and repeat purchases support dominance.
Children is projected to be the fastest-growing segment at a CAGR of 8.1% from 2025 to 2032, driven by demand for pediatric formulations such as syrups and low-dose tablets. Parents seek safe, effective treatments for colds, flu, and allergies. Increased access to pediatric healthcare in emerging regions is boosting adoption. Child-friendly liquid formulations are preferred for ease of administration. Awareness campaigns and OTC accessibility support growth. Rising pediatric population in Asia-Pacific contributes to the expansion of this segment.
- By Dosage Form
On the basis of dosage form, the phenylpropanolamine (PPA) market is segmented into solid dosage form, liquid dosage form, and others. Solid dosage form dominated with a share of 61.9% in 2024, due to stability, affordability, and convenience of tablets and capsules. Solid formulations are widely used for adult cold and allergy treatments. Their long shelf life and easy distribution reinforce adoption. OTC and prescription solid forms are highly accessible globally. Seasonal demand for tablets drives recurring sales. Pharmaceutical companies prioritize this dosage form for mass production and retail availability.
Liquid dosage form is expected to be the fastest-growing subsegment at a CAGR of 7.8% from 2025 to 2032, particularly due to demand for pediatric and geriatric medicines. Syrups and suspensions offer easier consumption for children and elderly patients. Liquid formulations provide rapid onset of action for acute symptoms. Combination liquids with multiple active ingredients are gaining traction. Expanding online and retail pharmacy channels support distribution. Liquid dosage growth is reinforced by increasing parental preference for safe and palatable medicines.
- By End User
On the basis of end user, the phenylpropanolamine (PPA) market is segmented into hospitals, specialty clinics, and others. Hospitals dominated with a share of 39.7% in 2024, as PPA continues to be used under medical supervision for respiratory, allergy, and weight management treatments. Hospitals serve as a primary channel for prescription and high-dose formulations. Their established infrastructure ensures compliance with safety regulations. Seasonal demand peaks further reinforce hospital reliance. Hospitals also contribute to bulk procurement and institutional sales. Physician oversight enhances trust and adoption.
Specialty clinics are projected to grow the fastest at a CAGR of 7.5% from 2025 to 2032, driven by outpatient management of allergies, respiratory conditions, and obesity treatments. Clinics provide targeted services and prescription guidance. Rising patient visits and healthcare awareness support adoption. Clinics often stock both OTC and prescription PPA products. Convenience for patients seeking specialist care boosts this segment. Expansion of private clinics in emerging markets accelerates growth.
- By Distribution Channel
On the basis of distribution channel, the phenylpropanolamine (PPA) market is segmented into hospital pharmacies, retail pharmacies, online pharmacies, and others. Retail pharmacies dominated with the largest share of 45.3% in 2024, reflecting consumer preference for easily accessible OTC medications. Retail networks provide widespread geographic coverage, ensuring product availability. Seasonal spikes in cold and allergy treatments further reinforce dominance. Consumers prefer immediate purchase and affordability. Marketing and promotions by pharmacy chains enhance visibility. Retail channels support both adult and pediatric formulations effectively.
Online pharmacies are expected to be the fastest-growing distribution channel at a CAGR of 9.2% from 2025 to 2032, driven by convenience, discounts, and home delivery services. Consumers increasingly purchase OTC and prescription medications online. Online platforms allow broader reach into remote areas and emerging markets. The trend toward e-pharmacies is accelerated by digital healthcare adoption. Easy comparison of prices and product availability attracts cost-conscious buyers. Online growth is further supported by rising smartphone and internet penetration globally.
Phenylpropanolamine (PPA) Market Regional Analysis
- North America dominated the phenylpropanolamine market with the largest revenue share of 38.1% in 2024, driven by advanced pharmaceutical manufacturing capabilities, established healthcare infrastructure, and consistent usage in both prescription and veterinary drugs, with the U.S. leading due to strong demand for cold and flu medications
- Consumers in the region prioritize effectiveness, safety, and accessibility, leading to strong adoption of PPA-based products for cold, flu, and allergy relief. The presence of major pharmaceutical manufacturers and regulatory compliance frameworks ensures high-quality formulations and steady supply
- This dominance is further supported by increasing healthcare expenditure, widespread availability of retail and online pharmacies, and a technologically inclined population that is comfortable with accessing medications through multiple channels, establishing PPA-based treatments as trusted solutions for human and veterinary applications in North America
U.S. Phenylpropanolamine (PPA) Market Insight
The U.S. phenylpropanolamine (PPA) market captured the largest revenue share of 42% in North America in 2024, driven by strong demand for cold, flu, and allergy treatments and the widespread availability of OTC and prescription medications. Consumers prioritize effective and safe decongestant and appetite suppressant formulations, leading to high adoption across both adult and pediatric populations. The presence of well-established pharmaceutical manufacturers, robust regulatory frameworks, and advanced healthcare infrastructure supports steady market growth. In addition, the growing prevalence of respiratory ailments and increased awareness of seasonal cold management is contributing to consistent demand. Retail and online pharmacies provide easy access, further strengthening market penetration.
Europe Phenylpropanolamine Market Insight
The Europe phenylpropanolamine (PPA) market is projected to grow at a substantial CAGR during the forecast period, fueled by rising cases of respiratory allergies, colds, and sinusitis, alongside the growing preference for safe and cost-effective OTC medications. Increasing urbanization and higher consumer awareness regarding health and wellness are fostering adoption. Pharmaceutical companies are focusing on compliant formulations to meet stringent EU regulatory standards. The market is witnessing growth across hospitals, clinics, and retail pharmacies, with both prescription and OTC segments contributing to the expansion. Consumers are also inclined towards combination therapies that offer multi-symptom relief, supporting segment growth.
U.K. Phenylpropanolamine Market Insight
The U.K. phenylpropanolamine (PPA) market is anticipated to grow at a noteworthy CAGR, driven by the rising prevalence of cold, flu, and allergy-related conditions, coupled with increasing consumer awareness of effective OTC treatments. Adults remain the primary user group, while pediatric formulations are gaining traction. The well-established healthcare infrastructure and easy access through retail and online pharmacies further support market expansion. Physicians and healthcare professionals are recommending safe PPA-based therapies for specific indications, enhancing adoption. The preference for convenient and quick-acting solid and liquid dosage forms is stimulating demand.
Germany Phenylpropanolamine Market Insight
The Germany phenylpropanolamine (PPA) market is expected to expand at a considerable CAGR, fueled by the country’s advanced healthcare infrastructure, high consumer awareness, and stringent safety regulations. The growing incidence of seasonal colds, allergies, and sinusitis drives demand for effective decongestant formulations. Both human and veterinary applications are witnessing adoption, with hospitals, specialty clinics, and retail pharmacies serving as key distribution channels. Pharmaceutical companies are introducing compliant and high-quality products, which align with local consumer expectations for safety and efficacy. Combination therapies and pediatric formulations are also gaining prominence.
Asia-Pacific Phenylpropanolamine Market Insight
The Asia-Pacific phenylpropanolamine (PPA) market is poised to grow at the fastest CAGR of 8.7% during 2025–2032, driven by rising population, urbanization, and increasing prevalence of respiratory ailments. Countries such as China, India, and Japan are witnessing strong demand for both OTC and prescription PPA-based medications. Expanding healthcare access, growing awareness of cold and allergy management, and the availability of affordable formulations are key growth factors. Emerging pharmaceutical manufacturing hubs in the region are enhancing supply and reducing costs. Increasing adoption in pediatric and adult populations, alongside rising demand for veterinary applications, further propels market growth.
Japan Phenylpropanolamine Market Insight
The Japan phenylpropanolamine (PPA) market is gaining momentum due to a high prevalence of seasonal respiratory illnesses and allergies, combined with consumer preference for safe and effective treatments. Solid and liquid dosage forms are widely used across adult and pediatric populations. Hospitals, specialty clinics, and retail pharmacies form the primary distribution channels. Increased health awareness and aging population trends are boosting demand for easily administrable medications. Combination therapies and reformulated safer products are also supporting market expansion. Integration of OTC accessibility with physician-guided prescriptions enhances market reach.
India Phenylpropanolamine Market Insight
The India phenylpropanolamine (PPA) market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to rapid urbanization, a growing middle class, and increasing healthcare expenditure. The country exhibits high demand for both OTC and prescription cold, allergy, and weight management medications. Expanding retail and online pharmacy networks improve accessibility and affordability. Growing awareness of respiratory ailments and seasonal cold management is driving adoption across adult and pediatric populations. Domestic pharmaceutical manufacturers are actively producing compliant PPA formulations, further supporting market growth. Government initiatives to improve healthcare access and availability of low-cost medications enhance the market landscape.
Phenylpropanolamine (PPA) Market Share
The Phenylpropanolamine (PPA) industry is primarily led by well-established companies, including:
- MALLADI. (India)
- ALPS Pharmaceutical Ind. Co. Ltd. (Japan)
- BIOTECHNICA DWC LLC (Taiwan)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Medisca Inc. (Canada)
- Panion and BF Biotech Inc. (Denmark)
- PCCA (U.S.)
- Vetoquinol (France)
- Sutphin Drugs Inc. (U.S.)
- Covetrus (U.S.)
- NW Compounders. (U.S.)
- PetMart Pharmacy (U.S.)
- PRN (U.S.)
- Clinivex (U.S.)
- Sigma-Aldrich (U.S.)
- Sanofi (France)
- Carinopharm GmbH (Germany)
- GSK plc (U.K.)
What are the Recent Developments in Global Phenylpropanolamine (PPA) Market?
- In February 2025, the veterinary publication DVM360 released a detailed article on PPA use in dogs and cats, confirming that it remains a "key treatment" for urinary incontinence, this demonstrates the continued, stable presence of PPA products such as Proin in the veterinary market. The article's focus on toxicology and its practical use for veterinarians signifies ongoing market activity and the drug's sustained relevance, which underpins the recent generic launch and other developments
- In June 2024, the U.S. Food and Drug Administration approved the first generic phenylpropanolamine hydrochloride chewable tablets for use in dogs. This approval aims to provide an effective treatment option for canine urinary incontinence due to urethral sphincter hypotonus. The move is expected to enhance accessibility and affordability of PPA-based treatments in veterinary care
- In March 2023, legal firms, including Seeger Weiss LLP, were involved in litigation concerning the use of phenylpropanolamine in various over-the-counter medications. These legal actions highlighted ongoing concerns and scrutiny over the safety and marketing of PPA-containing products
- In July 2022, phenylpropanolamine was classified as a List I chemical under federal law in the United States, indicating its potential use in the illicit production of amphetamines. Handlers of PPA are required to comply with specific regulations to prevent its misuse, reflecting ongoing regulatory oversight in both human and veterinary applications
- In April 2021, PRN Pharmacal introduced Proin ER, an extended-release formulation of phenylpropanolamine hydrochloride, for the management of urinary incontinence in dogs. This product offers a convenient dosing schedule, aiming to improve compliance and quality of life for pets suffering from this condition
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.