Global Poly Adp Ribose Polymerase Parp Inhibitors Market
Market Size in USD Billion
CAGR :
% 
 USD
10.00 Billion
USD
23.89 Billion
2024
2032
USD
10.00 Billion
USD
23.89 Billion
2024
2032
| 2025 –2032 | |
| USD 10.00 Billion | |
| USD 23.89 Billion | |
|
|
|
|
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Size
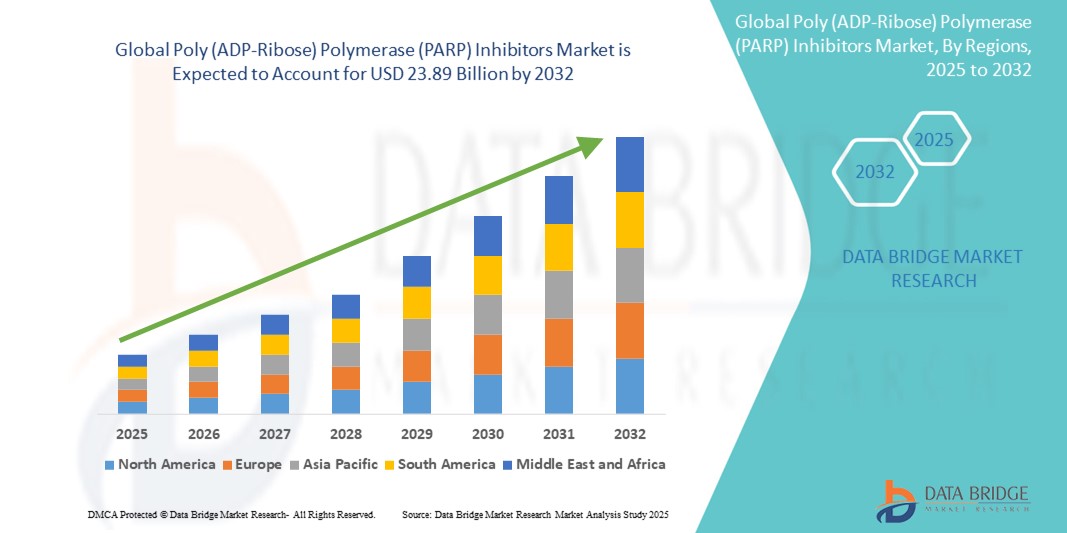
- The global Poly (ADP-Ribose) Polymerase (PARP) inhibitors market size was valued at USD 10.00 billion in 2024 and is expected to reach USD 23.89 billion by 2032, at a CAGR of 11.50% during the forecast period
- The market growth is largely fueled by the increasing prevalence of various types of cancer worldwide, particularly ovarian, breast, prostate, and pancreatic cancers, where PARP inhibitors have shown significant efficacy. This rising cancer burden, coupled with the growing demand for effective, targeted treatments, is driving the adoption of PARP inhibitors
- Furthermore, advancements in personalized medicine and biomarker research, specifically the identification of BRCA mutations and homologous recombination deficiency (HRD), are enabling more precise patient selection for PARP inhibitor therapies, thereby expanding their clinical utility
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Analysis
- Poly (ADP-ribose) Polymerase (PARP) inhibitors, acting as targeted cancer drugs, are increasingly vital components of modern oncology, particularly in the treatment of various cancers due to their ability to exploit cancer cells' DNA repair deficiencies
- The escalating demand for PARP inhibitors is primarily fueled by the rising global incidence of cancer, particularly ovarian, breast, prostate, and pancreatic cancers. In addition, the growing understanding of cancer genomics and the role of biomarkers in identifying patients who will benefit most from these therapies is a key driver
- North America dominated the Poly (ADP-Ribose) Polymerase (PARP) Inhibitors market with the largest revenue share of 44% in 2024, characterized by a high prevalence of target cancers, advanced healthcare infrastructure, significant research and development investments, and favorable regulatory environments. The region has seen substantial growth driven by both established treatments and emerging therapies, with a strong presence of key industry players
- Asia-Pacific is expected to be the fastest growing region in the Poly (ADP-Ribose) Polymerase (PARP) inhibitors market during the forecast period due to increasing urbanization, rising disposable incomes, improving healthcare infrastructure, and a growing cancer burden in countries such as China and India
- Breast Cancer segment dominated the Poly (ADP-Ribose) Polymerase (PARP) inhibitors market with a market share of 48.47% in 2024, driven by the high incidence of the disease, particularly BRCA-mutated breast cancer, and the significant efficacy demonstrated by PARP inhibitors as targeted therapy in this patient population
Report Scope and Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Segmentation
|
Attributes |
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Trends
“Enhanced Precision and Discovery Through AI Integration”
- A significant and accelerating trend in the global PARP inhibitors market is the deepening integration of artificial intelligence (AI) and machine learning (ML) across various stages of drug development and clinical application. This fusion of technologies is significantly enhancing the precision and efficiency of discovering, developing, and deploying PARP inhibitor therapies, ultimately improving patient outcomes.
- For instance, AI is being leveraged in the discovery and design of novel PARP inhibitors. Generative models trained on vast chemical databases can rapidly identify and design new small molecules with desired pharmacological properties, potentially accelerating the development pipeline
- AI integration in the PARP inhibitor market also enables features such as learning patient response patterns to suggest optimal treatment regimens and providing more intelligent insights based on real-world data. For instance, AI can analyze multimodal patient data to predict treatment outcomes and identify patients most such asly to benefit from PARP inhibitor therapy. Furthermore, AI can aid in streamlining clinical trials by optimizing patient recruitment, monitoring adverse events, and analyzing trial data more efficiently
- The seamless integration of AI with broader oncology platforms facilitates centralized control over various aspects of cancer treatment. Through a single interface, clinicians can access AI-driven insights to inform treatment decisions, manage patient data, and monitor therapy effectiveness, creating a unified and data-driven approach to cancer care
- This trend towards more intelligent, precise, and data-driven PARP inhibitor development and application is fundamentally reshaping expectations for cancer therapy. Consequently, pharmaceutical companies and biotech firms are increasingly investing in AI capabilities to accelerate drug discovery, improve clinical trial efficiency, and refine patient stratification for PARP inhibitor therapies
- The demand for PARP inhibitors that are supported by AI-driven insights is growing rapidly across the oncology sector, as clinicians and researchers increasingly prioritize precision, efficacy, and optimized patient selection in cancer treatment
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Dynamics
Driver
“Growing Need Due to Rising Cancer Incidence and Advancements in Precision Oncology”
- The increasing global prevalence of various cancer types, particularly those with homologous recombination deficiency (HRD) such as ovarian, breast, prostate, and pancreatic cancers, coupled with the accelerating advancements in personalized medicine and genomic testing, is a significant driver for the heightened demand for PARP inhibitors
- For instance, ongoing research and development by pharmaceutical companies such as AstraZeneca, Merck, and GSK, and strategic collaborations, are continually expanding the indications and efficacy of PARP inhibitors. Such efforts are expected to drive significant growth in the PARP inhibitor market in the forecast period
- As the understanding of cancer genomics deepens and clinicians become more aware of the importance of identifying specific genetic mutations (such as BRCA1/2) in patients, PARP inhibitors offer a targeted and highly effective treatment option. These drugs exploit the DNA repair vulnerabilities in cancer cells, providing a compelling therapeutic approach for patients with specific genetic profiles
- Furthermore, the growing popularity of personalized medicine and the desire for tailored treatment plans are making PARP inhibitors an integral component of modern oncology. They offer a precise approach that aligns with individual patient characteristics, improving outcomes and reducing side effects compared to traditional chemotherapy
- The increasing accessibility of genomic testing, combined with the continuous discovery of new biomarkers, further propels the adoption of PARP inhibitors in the treatment of a wider range of cancers. The trend towards combination therapies, where PARP inhibitors are used alongside other anticancer agents, further contributes to market growth by enhancing therapeutic efficacy
Restraint/Challenge
“Drug Resistance Development and High Treatment Costs”
- A significant challenge to broader market penetration for PARP inhibitors is the development of drug resistance in cancer cells, alongside the relatively high cost of treatment. While PARP inhibitors initially show promising results, cancer cells often develop mechanisms to circumvent their effects over time, limiting long-term efficacy and leading to disease progression. This acquired resistance is a major concern for both patients and healthcare providers
- For instance, studies have identified various mechanisms of resistance, such as secondary mutations in BRCA genes that restore DNA repair function, increased drug efflux, or changes in other DNA repair pathways. These biological hurdles necessitate continuous research into overcoming resistance and developing novel combination therapies
- Addressing these challenges requires substantial investment in biomarker discovery to better select patients who will respond to therapy and in research to understand and counteract resistance mechanisms
- Furthermore, the high initial cost of PARP inhibitor therapies poses a significant barrier to access, particularly in regions with limited healthcare budgets or for patients without adequate insurance coverage. While these drugs offer significant clinical benefits, their premium pricing can limit widespread adoption and create financial burdens on healthcare systems and individuals
- Overcoming these challenges through intensive research into resistance mechanisms, development of more accessible and affordable formulations, and robust payer negotiations for reimbursement will be vital for sustained market growth and ensuring wider patient access to these life-saving therapies
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Scope
The market is segmented on the basis of drug type, indication type, end users, and distribution channel
- By Drug Type
On the basis of drug type, the Poly (ADP-Ribose) Polymerase (PARP) inhibitors market is segmented into talazoparib, veliparib, olaparib, and others. The Olaparib segment dominated the market with the largest revenue share in 2024, owing to its broad clinical approvals and first-mover advantage in the treatment of multiple cancers including ovarian, breast, prostate, and pancreatic cancers. Olaparib’s extensive use in maintenance therapy, coupled with favorable reimbursement policies and ongoing clinical trials, supports its sustained market dominance.
The Talazoparib segment is projected to register the fastest CAGR from 2025 to 2032, driven by its high potency and emerging use in combination therapies. Its approval for BRCA-mutated breast cancer and favorable results in pipeline indications provide significant growth momentum.
- By Indication type
On the basis of indication type, the Poly (ADP-Ribose) Polymerase (PARP) inhibitors market is segmented into ovarian cancer, fallopian tube cancer, breast cancer, and others. The Breast Cancer segment dominated the Poly (ADP-Ribose) Polymerase (PARP) Inhibitors market with a market share of 48.47% in 2024, attributed to the high prevalence of BRCA mutations among breast cancer patients and increasing adoption of PARP inhibitors such as Olaparib and Talazoparib for BRCA-mutated HER2-negative metastatic breast cancer. Favorable clinical outcomes, expanded regulatory approvals, and improved awareness of genetic testing continue to drive the segment’s strong performance.
The Ovarian Cancer segment is expected to witness fastest growth during 2025 to 2032, supported by the well-established role of PARP inhibitors in frontline and maintenance therapies. Ongoing research on combination therapies and greater access to genetic diagnostics contribute to sustained market relevance.
- By End User
On the basis of end users, the Poly (ADP-Ribose) Polymerase (PARP) inhibitors market is segmented into hospitals, homecare, specialty clinics, and others. The Hospitals segment dominated the market in 2024, due to the presence of specialized oncology departments, availability of skilled professionals, and insurance coverage for cancer therapies. Hospitals are primary points for initial diagnosis and treatment administration, especially for advanced-stage cancers.
The Homecare segment is anticipated to grow at the fastest pace during forecast period, driven by the shift toward oral formulations and outpatient cancer care. Increasing preference for at-home treatment options to improve patient comfort and reduce healthcare costs further accelerates this trend.
- By Distribution Channel
On the basis of distribution channel, the Poly (ADP-Ribose) Polymerase (PARP) inhibitors market is segmented into Hospital Pharmacy, Online Pharmacy, Retail Pharmacy, and Others. Hospital pharmacies accounted for the largest market share in 2024, as most prescriptions are initiated in hospital settings post-diagnosis. The close linkage between oncology treatment centers and in-house pharmacies ensures prompt access to specialized medications.
Online pharmacies are expected to register the highest growth rate from 2025 to 2032, propelled by increasing digital penetration, rising preference for doorstep delivery of chronic medications, and favorable e-commerce policies. The convenience of online reordering and subscription models for long-term treatment regimens further enhances segment growth.
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Regional Analysis
- North America dominated the Poly (ADP-Ribose) Polymerase (PARP) inhibitors market with the largest revenue share of 44% in 2024, driven by high prevalence of target cancers, advanced healthcare infrastructure, significant research and development investments, and favorable regulatory environments
- Consumers and healthcare providers in the region highly value the efficacy, targeted approach, and established clinical benefits offered by PARP inhibitors for various cancer types, particularly those with BRCA mutations
- This widespread adoption is further supported by high disposable incomes, significant investments in pharmaceutical R&D, a robust regulatory framework (such as the FDA approvals), and widespread access to genomic testing. These factors collectively establish PARP inhibitors as a favored solution for precision oncology in both academic and community cancer centers across North America
U.S. Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Insight
The U.S. Poly (ADP-Ribose) Polymerase (PARP) inhibitors market captured the largest revenue share within North America in 2024, fueled by the swift uptake of targeted oncology therapies and the expanding trend of precision medicine. Healthcare providers and patients are increasingly prioritizing the enhancement of cancer treatment outcomes through intelligent, biomarker-driven therapeutic approaches. The growing preference for personalized medicine, combined with robust demand for advanced diagnostic testing (and integration into comprehensive cancer care pathways, further propels the PARP inhibitor industry. Moreover, the increasing integration of cutting-edge genomic technologies and supportive regulatory frameworks is significantly contributing to the market's expansion.
Europe Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Insight
The Europe Poly (ADP-Ribose) Polymerase (PARP) inhibitors market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by the escalating need for enhanced cancer treatment outcomes and the growing adoption of personalized medicine in clinical practice. The increase in cancer incidence, coupled with the demand for targeted therapies, is fostering the adoption of PARP inhibitors. European healthcare systems are also drawn to the efficacy and specificity these drugs offer. The region is experiencing significant growth across various cancer indications, with PARP inhibitors being incorporated into both established treatment guidelines and emerging therapeutic strategies.
U.K. Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Insight
The U.K. Poly (ADP-Ribose) Polymerase (PARP) inhibitors market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the escalating trend of personalized cancer therapy and a desire for heightened treatment efficacy and patient outcomes. In addition, concerns regarding cancer recurrence and the need for durable responses are encouraging both clinicians and patients to choose targeted PARP inhibitor solutions. The U.K.'s embrace of advanced oncology practices, alongside its robust healthcare infrastructure and strong research ecosystem, is expected to continue to stimulate market growth.
Germany Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Insight
The Germany Poly (ADP-Ribose) Polymerase (PARP) inhibitors market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of precision medicine and the demand for technologically advanced, efficacious cancer solutions. Germany’s well-developed healthcare infrastructure, combined with its emphasis on innovation and evidence-based medicine, promotes the adoption of PARP inhibitors, particularly in specialized oncology centers. The integration of PARP inhibitors with comprehensive cancer care pathways is also becoming increasingly prevalent, with a strong preference for secure, data-driven treatment options aligning with local patient and clinician expectations.
Asia-Pacific Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Insight
The Asia-Pacific Poly (ADP-Ribose) Polymerase (PARP) inhibitors market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032. This growth is driven by increasing cancer incidence, rising disposable incomes, and technological advancements in healthcare in countries such as China, Japan, and India. The region's growing inclination towards precision oncology, supported by government initiatives promoting cancer care infrastructure and genomic testing, is driving the adoption of PARP inhibitors. Furthermore, as APAC emerges as a hub for pharmaceutical manufacturing and clinical trials, the affordability and accessibility of PARP inhibitors are expanding to a wider patient base
Japan Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Insight
The Japan Poly (ADP-Ribose) Polymerase (PARP) inhibitors market is gaining momentum due to the country’s high-tech healthcare culture, increasing cancer burden, and demand for innovative therapies. The Japanese market places a significant emphasis on evidence-based medicine and comprehensive patient care, and the adoption of PARP inhibitors is driven by the increasing number of diagnosed cancer cases and advancements in genetic testing. The integration of PARP inhibitors with other systemic therapies and ongoing research into new indications is fueling growth. Moreover, Japan's aging population is such asly to spur demand for highly effective, targeted cancer solutions in both academic and community oncology settings.
India Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Insight
The India Poly (ADP-Ribose) Polymerase (PARP) inhibitors market accounted for a substantial market revenue share in Asia Pacific in 2024, attributed to the country's expanding middle class, rapid urbanization, and high rates of cancer incidence. India stands as a rapidly growing market for advanced oncology treatments, and PARP inhibitors are becoming increasingly popular in major cancer centers and private hospitals. The push towards improving cancer care infrastructure and the increasing availability of genomic testing, alongside a growing number of domestic and international pharmaceutical players, are key factors propelling the market in India.
Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market Share
The Poly (ADP-Ribose) Polymerase (PARP) inhibitors industry is primarily led by well-established companies, including:
- AstraZeneca (U.K.)
- Merck & Co., Inc., (U.S.)
- GSK plc. (U.K.)
- Pfizer Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Bristol-Myers Squibb (U.S.)
- BeiGene (U.S.)
- Repare Therapeutics (U.S.)
- Novartis AG (Switzerland)
- Bayer AG (Germany)
- Zentalis Pharmaceuticals (U.S.)
- Jiangsu Hengrui Pharmaceuticals (China)
- Artios Pharma (U.K.)
- Karyopharm Therapeutics Inc. (U.S.)
- Ono Pharmaceutical Co. Ltd. (Japan)
- Idience Co., Ltd. (South Korea)
- IMPACT Therapeutics (China)
- Allarity Therapeutics (U.S.)
What are the Recent Developments in Global Poly (ADP-Ribose) Polymerase (PARP) Inhibitors Market?
- In June 2025, Johnson & Johnson announced first results from the Phase 3 AMPLITUDE study, evaluating the combination of niraparib and abiraterone acetate plus prednisone (AAP) in patients with metastatic castration-sensitive prostate cancer (mCSPC) with homologous recombination repair (HRR) alterations. The study met its primary endpoint of radiographic progression-free survival (rPFS), showing a clinically meaningful and statistically significant improvement
- In June 2025, Scientists at The University of Texas Health Science Center at San Antonio (UT Health San Antonio) announced a significant discovery in June 2025 regarding a key DNA complex (CST complex) connected to PARP inhibitor resistance, opening doors for new strategies. In addition, studies are ongoing into PARP inhibitor rechallenge strategies, with promising results from trials such as KGOG 3056/NIRVANA-R (NCT04734665) presented at the 2025 SGO Annual Meeting on Women's Cancer
- In February 2025, A comprehensive review published reaffirmed Olaparib's leadership among PARP inhibitors in boosting overall survival for platinum-sensitive recurrent ovarian cancer, indicating its potential as a preferred option for patients
- In May 2024, The Re-VOLVE Phase II clinical trial (NCT05065021), presented in May 2025, investigates a chemo-free approach for women with ovarian cancer progressing post-PARP inhibitor treatment, adapting therapy to real-time assessment of evolving genomic resistance. This highlights the ongoing efforts to personalize PARP inhibitor treatment and address resistance
- In June 2023, Ariceum Therapeutics, a private biotech firm focused on radiopharmaceutical innovations for challenging cancers, announced its acquisition of Theragnostics Ltd. This UK-based biopharmaceutical company specializes in developing radio-labeled PARP inhibitors aimed at diagnosing and treating tumors. The acquisition is expected to enhance Ariceum's portfolio and accelerate advancements in targeted cancer therapies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.