Global Prader Willi Syndrome Drug Market
Market Size in USD Billion
CAGR :
% 
 USD
1.12 Billion
USD
1.39 Billion
2024
2032
USD
1.12 Billion
USD
1.39 Billion
2024
2032
| 2025 –2032 | |
| USD 1.12 Billion | |
| USD 1.39 Billion | |
|
|
|
|
Prader-Willi Syndrome Drug Market Size
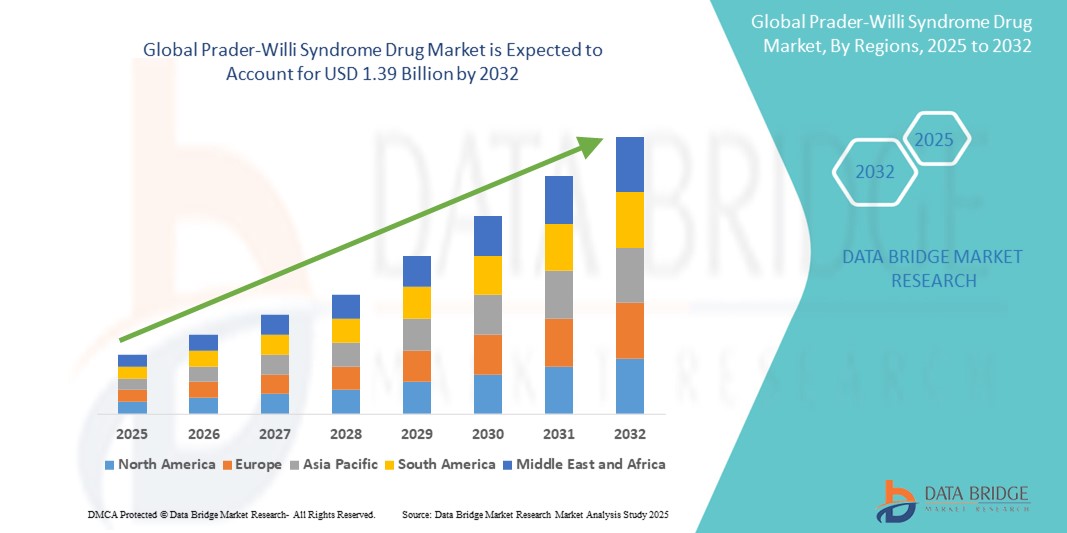
- The global Prader-Willi Syndrome Drug market was valued at USD 1.12 billion in 2024 and is expected to reach USD 1.39 billion by 2032 at a CAGR of 2.75%, during the forecast period
- This growth is driven by factors the increase in the health consciousness among population across the globe
Prader-Willi Syndrome Drug Market Analysis
- Prader-Willi Syndrome (PWS) is a complex, rare genetic disorder characterized by hypotonia, insatiable appetite, developmental delays, cognitive disabilities, and behavioral issues. It is primarily caused by the loss of function of specific genes on chromosome 15. Early diagnosis and management are crucial to improving long-term outcomes for individuals affected by this condition
- The surge in awareness of rare genetic disorders, combined with advancements in genetic testing and research, has accelerated the identification and management of Prader-Willi Syndrome. Increased focus on early therapeutic intervention, growth hormone therapy, and appetite suppressants is further boosting the market
- North America is expected to dominate the Prader-Willi Syndrome drug market with a share of 44.67%, due to early adoption of growth hormone treatments, advanced genetic diagnostic capabilities, and strong presence of key pharmaceutical players.
- For instance, the increasing number of patients diagnosed through newborn screening programs and receiving early growth hormone therapy has contributed significantly to North America’s leadership in PWS drug development and care.
- Asia-Pacific is expected to be the fastest growing region in the Prader-Willi Syndrome drug market during the forecast period due to improving healthcare infrastructure, increased adoption of genetic screening, and rising prevalence awareness in emerging economies
- The genotropin segment is expected to dominate the market with a market share of 39.12% due to its widespread use in managing growth deficiency in PWS patients. Omnitrope and Norditropin are also gaining momentum as effective recombinant human growth hormone therapies
Report Scope and Prader-Willi Syndrome Drug Market Segmentation
|
Attributes |
Prader-Willi Syndrome Drug Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
• Johnson & Johnson (U.S.) • Novo Nordisk A/S (Denmark) • Pfizer Inc. (U.S.) • Levo Therapeutics (U.S.) • Rhythm Pharmaceuticals, Inc. (U.S.) • Ferring Pharmaceuticals (Switzerland) • Sanofi (France) • Soleno Therapeutics (U.S.) • Millendo Therapeutics, Inc. (U.S.) • Ipsen (France) • Teva Pharmaceutical Industries Ltd. (Israel) • Zafgen, Inc. (U.S.) • Merck & Co., Inc. (U.S.) • Novartis AG (Switzerland) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Prader-Willi Syndrome Drug Market Trends
“Growing Preference for Natural and Organic Formulations”
- One prominent trend in the Prader-Willi Syndrome Drug market is the increasing caregiver and patient preference for natural and organic therapeutic options to manage symptoms such as anxiety, sleep disorders, and behavioral disturbances.
- These formulations, often enriched with botanical extracts such as valerian root, passionflower, chamomile, and melatonin-based natural supplements, offer calming and regulatory effects without severe side effects, appealing to the growing segment of health-conscious consumers and caregivers seeking holistic care approaches
For instance,
- the demand for clean-label, non-synthetic, and plant-based therapies is driving R&D efforts and pushing pharmaceutical and nutraceutical manufacturers to expand their portfolios with certified organic, non-GMO, and additive-free formulations
This trend is transforming the landscape of Prader-Willi Syndrome drug treatments, as both pharmaceutical and wellness companies increasingly blend therapeutic efficacy with natural wellness benefits to improve long-term adherence, caregiver trust, and patient quality of life
Prader-Willi Syndrome Drug Market Dynamics
Driver
‘Growing Need Due to Rising Awareness and Diagnosis Rates of Rare Genetic Conditions”
- The increasing recognition and diagnosis of Prader-Willi Syndrome globally is significantly contributing to the growing demand for targeted treatment options.
- As awareness improves among healthcare providers and caregivers, more children are being diagnosed at earlier stages, leading to early therapeutic intervention and better disease management.
- The complex nature of PWS, encompassing hyperphagia, developmental delays, and behavioral issues, necessitates a multifaceted pharmacological approach that includes growth hormone therapy, appetite suppressants, and psychotropic medications.
- Additionally, government and non-profit initiatives to support rare disease research are accelerating clinical development of novel therapies.
For instance,
- In August 2023, according to the Foundation for Prader-Willi Research, early diagnosis through genetic testing has increased significantly over the past decade, especially in high-income regions, resulting in improved access to supportive therapies and clinical trials.
- In December 2022, a report by Orphanet noted a 40% increase in the registration of PWS patients in global rare disease registries, highlighting the growing visibility and prioritization of this condition in medical research.
- As diagnosis rates rise and patient advocacy gains momentum, the demand for tailored drug solutions is expected to drive significant growth in the PWS drug market.
Opportunity
“Innovative Therapeutic Approaches Leveraging Epigenetic and Peptide-Based Technologies”
- Emerging therapies are focusing on novel biological targets such as ghrelin signaling pathways and oxytocin receptor modulation, which directly affect appetite regulation and social behaviors in PWS patients.
- Peptide-based drugs and epigenetic modulators offer promising avenues for long-term symptom management and improved quality of life.
For instance, - In February 2025, according to a publication in Nature Reviews Drug Discovery, several biotech firms initiated trials for oxytocin analogs and ghrelin antagonists that show promising results in appetite suppression and behavioral stabilization among PWS patients.
- In September 2024, research from the European Society of Human Genetics highlighted the potential of CRISPR/Cas9-based therapies in reactivating the maternal UBE3A gene—dormant in PWS—offering a potential disease-modifying strategy.
- These innovations are expected to unlock new opportunities for drug developers and significantly impact treatment paradigms for Prader-Willi Syndrome.
Restraint/Challenge
“High Cost of Drug Development for Ultra-Rare Disorders Limiting Market Accessibility”
- The high cost associated with the research, development, and commercialization of therapies for ultra-rare conditions such as Prader-Willi Syndrome remains a major challenge.
- Due to small patient populations and complex clinical endpoints, developing effective treatments often requires substantial investment with limited return on investment, deterring pharmaceutical interest.
For instance,
- In November 2024, according to an article published by Global Genes, the average cost of bringing a rare disease therapy to market can exceed USD 1 billion, significantly higher than for more common conditions.
- A 2023 report by Evaluate Pharma stated that the limited commercial potential of orphan drugs in ultra-rare indications often restricts patient access due to high per-treatment pricing and limited insurance coverage.
- As a result, this financial barrier hinders broader market penetration, delays regulatory approvals, and creates disparities in global access to cutting-edge treatments.
Prader-Willi Syndrome Drug Market Scope
The market is segmented on the basis of drugs, diagnosis, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Drugs |
|
|
By Diagnosis |
|
|
By End User |
|
|
By Distribution Channel
|
|
In 2025, the Genotropin segment is projected to dominate the Herpes Zoster Drug Market with the largest share in the drug type segment.
The genotropin segment is expected to hold the largest market share of approximately 38.65% in 2025 due to its human growth hormone, is widely prescribed to manage growth failure and improve physical development in patients with Prader-Willi Syndrome (PWS). The drug’s efficacy in enhancing height, muscle tone, and metabolism, along with its FDA approval for pediatric growth hormone deficiency including PWS, reinforces its leading position. Increasing awareness among healthcare providers and caregivers, as well as strong clinical data supporting Genotropin’s long-term benefits, are key drivers behind its dominance
The genetic testing segment is expected to account for the largest share during the forecast period within the diagnosis segment.
In 2025, genetic testing are anticipated to dominate due to its ability to detect the underlying chromosomal abnormalities, including deletions and imprinting defects on chromosome 15.Growing accessibility to advanced diagnostic tools, rising awareness of rare genetic disorders, and increasing demand for early and accurate diagnosis are supporting the expansion of this segment. Improved reimbursement policies and precision medicine initiatives further strengthen the role of genetic testing in the diagnostic landscape of PWS
Prader-Willi Syndrome Drug Market Regional Analysis
“North America is the Dominant Region in the Prader-Willi Syndrome Drug Market”
- North America dominates the Prader-Willi Syndrome Drug market, driven by a well-established healthcare infrastructure, high awareness regarding rare genetic disorders, and the strong presence of leading pharmaceutical companies focused on orphan diseases.
- The U.S. holds a significant share due to increasing diagnosis rates of Prader-Willi Syndrome, extensive research funding, and the availability of approved therapies such as growth hormone treatments and appetite suppressants.
- The presence of specialized care centers, genetic counseling services, and active support organizations such as the Foundation for Prader-Willi Research further accelerates market growth in this region.
- Additionally, continuous clinical trial activity, the development of novel drug formulations, and strong regulatory incentives under the Orphan Drug Act are contributing to the expansion of the Prader-Willi Syndrome Drug market across North America.
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is expected to witness the fastest growth in the Prader-Willi Syndrome Drug market due to rising awareness about rare diseases, increasing healthcare investments, and improving diagnostic capabilities.
- Countries such as India, China, and Japan are emerging as key markets, driven by growing medical genetics infrastructure, increasing inclusion of rare diseases in national healthcare agendas, and rising patient advocacy efforts.
- Japan, known for its innovation in biotechnology and medical research, remains a crucial market with government-supported rare disease programs and advanced therapeutic development.
- In India and China, the expansion of genetic screening initiatives, increasing availability of specialized treatment centers, and the involvement of both domestic and international pharmaceutical players offering cost-effective therapies continue to boost market penetration.
Prader-Willi Syndrome Drug Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Johnson & Johnson (U.S.)
- Novo Nordisk A/S (Denmark)
- Pfizer Inc. (U.S.)
- Levo Therapeutics (U.S.)
- Rhythm Pharmaceuticals, Inc. (U.S.)
- Ferring Pharmaceuticals (Switzerland)
- Sanofi (France)
- Soleno Therapeutics (U.S.)
- Millendo Therapeutics, Inc. (U.S.)
- Ipsen (France)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Zafgen, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
Latest Developments in Global Prader-Willi Syndrome Drug Market
- In January 2025, Soleno Therapeutics announced positive Phase 3 results for DCCR (diazoxide choline controlled-release), its lead candidate for the treatment of hyperphagia and other symptoms in Prader-Willi Syndrome.
- In October 2024, Levo Therapeutics received orphan drug designation from the U.S. FDA for LV-101 (intranasal carbetocin), designed to improve social and behavioral symptoms associated with PWS.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.