Global Pyruvate Kinase Pk Deficiency Market
Market Size in USD Million
CAGR :
% 
 USD
450.50 Million
USD
675.80 Million
2024
2032
USD
450.50 Million
USD
675.80 Million
2024
2032
| 2025 –2032 | |
| USD 450.50 Million | |
| USD 675.80 Million | |
|
|
|
|
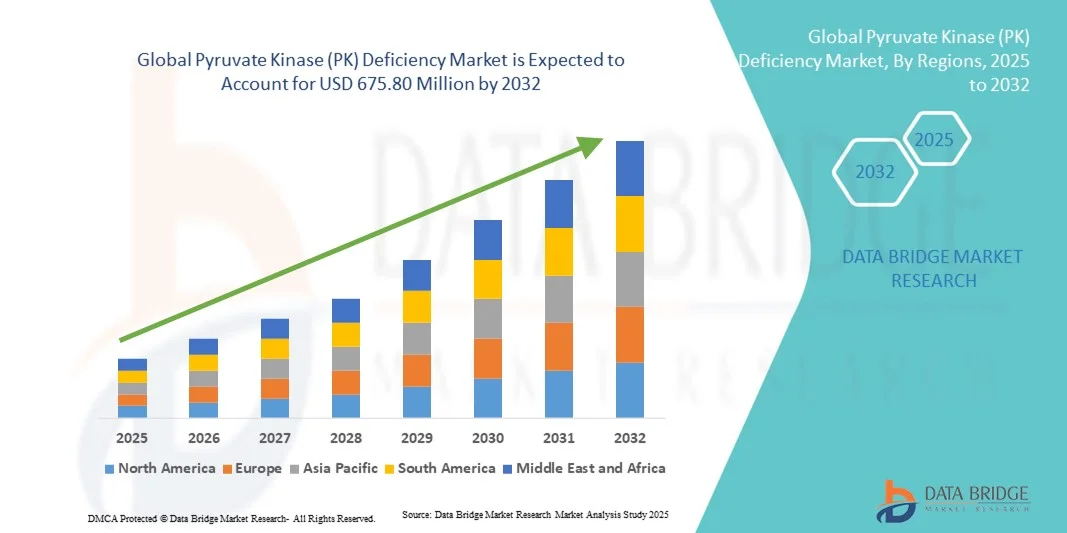
Pyruvate Kinase (PK) Deficiency Market Size
- The global pyruvate kinase (PK) deficiency market size was valued at USD 450.50 million in 2024 and is expected to reach USD 675.80 million by 2032, at a CAGR of 5.20% during the forecast period
- The market growth is primarily driven by increasing awareness, advancements in diagnostic techniques, and the development of novel therapies targeting PK deficiency, enhancing disease management and patient outcomes
- In addition, the rising prevalence of inherited hemolytic anemias and growing investment in rare disease research are fueling demand for effective and targeted treatment options. These factors are collectively propelling the adoption of innovative PK deficiency therapies, thereby significantly boosting the market’s growth
Pyruvate Kinase (PK) Deficiency Market Analysis
- Pyruvate kinase (PK) deficiency, a rare inherited disorder causing chronic hemolytic anemia, is increasingly recognized as a critical area for targeted therapeutic intervention due to its impact on patient quality of life and the need for specialized treatment strategies in both pediatric and adult populations
- The rising demand for PK deficiency treatments is primarily driven by growing awareness of rare hematologic disorders, advances in genetic testing and diagnostics, and the development of novel therapies that improve patient outcomes and reduce disease complications
- North America dominated the global pyruvate kinase (PK) deficiency market with the largest revenue share of 42.8% in 2024, attributed to advanced healthcare infrastructure, high adoption of innovative diagnostics and therapies, strong presence of key pharmaceutical players, and increased investment in rare disease research, particularly in the U.S.
- Asia-Pacific is expected to be the fastest-growing region in the global pyruvate kinase (PK) deficiency market during the forecast period due to increasing healthcare access, rising awareness about rare diseases, and growing government initiatives supporting early diagnosis and treatment of PK deficiency
- Bone Marrow Transplantation dominated the global pyruvate kinase (PK) deficiency market in 2024 with a market share of 29.6%, driven by its effectiveness in managing severe cases of PK deficiency and improving long-term patient outcomes, coupled with ongoing advancements in transplantation techniques and supportive care
Report Scope and Pyruvate Kinase (PK) Deficiency Market Segmentation
|
Attributes |
Pyruvate Kinase (PK) Deficiency Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Pyruvate Kinase (PK) Deficiency Market Trends
Advancements in Gene Therapy and Targeted Treatments
- A significant and accelerating trend in the global PK deficiency market is the growing development and adoption of gene therapies and enzyme activators such as mitapivat, which aim to address the underlying enzyme deficiency rather than just manage symptoms
- For instance, Mitapivat, a first-in-class pyruvate kinase activator, has shown promising clinical results in improving hemoglobin levels and reducing transfusion dependency in adult PK deficiency patients
- Novel therapies are increasingly personalized based on patient genotype and disease severity, enabling more effective and targeted management while potentially improving long-term outcomes
- The integration of real-world patient data and digital health platforms allows clinicians to monitor therapy response more closely, adjust treatments in real time, and optimize disease management strategies
- This trend towards precision medicine and targeted interventions is fundamentally reshaping treatment paradigms, driving patient and clinician preference for innovative therapies over traditional supportive care
- The demand for therapies that offer curative potential or significant reduction in disease burden is growing rapidly across both pediatric and adult populations, as patients seek long-term solutions rather than repetitive supportive interventions
Pyruvate Kinase (PK) Deficiency Market Dynamics
Driver
Increasing Awareness and Rare Disease Research Investment
- The rising prevalence of rare hematologic disorders and increasing awareness among healthcare professionals and patients is a significant driver for the PK deficiency market growth
- For instance, in March 2024, Agios Pharmaceuticals reported expanded clinical trials for mitapivat in pediatric populations, reflecting increasing investment in rare disease research and therapy development
- Growing knowledge of genetic testing and early diagnosis allows timely intervention, improving patient outcomes and fueling adoption of advanced therapies
- Furthermore, the expansion of rare disease networks and patient advocacy programs is facilitating faster access to treatment and increasing patient engagement
- Increasing availability of specialized treatment centers and hematology clinics is improving access to diagnosis and treatment, especially in developed regions with advanced healthcare infrastructure
- Growing collaboration between hospitals, research organizations, and pharmaceutical companies is accelerating knowledge sharing and enabling faster clinical implementation of innovative therapies
- Enhanced government funding and incentives for rare disease drug development further encourage pharmaceutical companies to innovate and expand their PK deficiency treatment pipelines, driving overall market growth
Restraint/Challenge
High Treatment Costs and Limited Global Access
- The high cost of advanced PK deficiency therapies and limited availability of specialized treatment centers pose significant challenges to broader market adoption
- For instance, gene therapy and mitapivat treatments can cost several hundred thousand dollars per patient annually, limiting access in developing regions or for uninsured patients
- Limited awareness among clinicians in emerging markets also restricts early diagnosis and treatment initiation, delaying optimal care for patients
- In addition, stringent regulatory approval processes and the need for extensive clinical trial data create barriers to faster market entry for new therapies
- Geographic disparities in healthcare infrastructure result in unequal access to advanced diagnostic tools and treatment modalities, particularly in low- and middle-income countries
- Patient adherence challenges, such as the requirement for frequent monitoring and follow-up, can affect treatment outcomes and limit the perceived effectiveness of novel therapies
- Addressing these challenges through improved insurance coverage, patient assistance programs, and global awareness campaigns will be critical for expanding access and sustaining market growth
Pyruvate Kinase (PK) Deficiency Market Scope
The market is segmented on the basis of population, diagnosis, treatment, end user, and distribution channel.
- By Population
On the basis of population, the global pyruvate kinase (PK) deficiency market is segmented into infants, children, and adults. The adults segment dominated the market with the largest revenue share of 51.2% in 2024, driven by the higher prevalence of diagnosed cases in adult populations and the greater uptake of novel therapies such as mitapivat. Adult patients often require long-term disease management strategies such as enzyme activators, transfusions, and supportive care, which contributes to sustained market revenue. The availability of specialized hematology clinics and better healthcare access in adult care settings further supports this dominance. In addition, clinical trials for advanced treatments predominantly include adult cohorts, enhancing adoption and awareness. The segment also benefits from growing awareness of PK deficiency among physicians treating hemolytic anemias, increasing timely diagnosis and treatment initiation.
The infants segment is expected to witness the fastest growth rate of 24.1% from 2025 to 2032, fueled by increased screening programs for newborns and early diagnosis initiatives. Infants diagnosed early can benefit from prompt interventions such as phototherapy, folic acid supplementation, and transfusions to prevent severe complications. The growing emphasis on pediatric rare disease management and parental awareness is contributing to higher therapy adoption. Advancements in neonatal diagnostics, including genetic testing, are driving early identification and treatment in infants. Furthermore, expanding healthcare infrastructure and pediatric hematology units in emerging markets are supporting faster growth. Early intervention in infants is increasingly recognized as critical for improving long-term patient outcomes and reducing disease burden.
- By Diagnosis
On the basis of diagnosis, the global pyruvate kinase (PK) deficiency market is segmented into bilirubin in the blood, complete blood count (CBC), genetic testing, haptoglobin blood test, osmotic fragility, pyruvate kinase activity, and stool urobilinogen. The genetic testing segment dominated with a market share of 34.5% in 2024 due to its critical role in accurate and early detection of PK deficiency. Genetic testing allows physicians to identify specific PKLR gene mutations, enabling personalized treatment strategies and better disease management. It is often used alongside enzyme activity assays to confirm diagnosis and guide therapeutic decisions. The rising availability of genetic testing facilities in hospitals and specialized clinics has improved adoption rates. Furthermore, payer coverage for genetic tests in developed regions is facilitating broader accessibility. Genetic testing is also increasingly integrated into newborn screening programs, driving consistent demand.
The pyruvate kinase activity assay segment is expected to witness the fastest growth rate of 27.6% from 2025 to 2032, driven by its role in directly assessing enzyme functionality and severity of hemolysis. This test allows real-time monitoring of disease progression and therapy response, particularly for novel activator treatments. Increased clinician awareness about the assay’s utility and improved laboratory infrastructure are boosting adoption. The segment also benefits from rapid advances in automated and high-throughput testing technologies, improving turnaround time and accuracy. Enhanced integration with genetic diagnostics further strengthens its relevance in personalized disease management. Rising demand for precise, actionable diagnostic information is expected to accelerate growth in this segment across all regions.
- By Treatment
On the basis of treatment, the global pyruvate kinase (PK) deficiency market is segmented into blood transfusion, bone marrow transplantation, splenectomy, phototherapy, folic acid, vitamin B, iron chelation (Key-Lay-Shun), cholecystectomy, and allogeneic hematopoietic stem cell transplantation (HSCT). The bone marrow transplantation segment dominated in 2024 with a market share of 29.6%, due to its curative potential for severe PK deficiency cases and long-term clinical outcomes. Bone marrow transplantation is particularly preferred in patients with high transfusion dependency or severe hemolytic anemia unresponsive to standard therapy. The segment benefits from advances in donor matching, conditioning regimens, and post-transplant supportive care, reducing complications and mortality. Hospital-based adoption is high due to the need for specialized care units and trained hematology teams. Increased clinical awareness and guidelines recommending transplantation for select severe cases further support market dominance. The segment also sees steady growth from adult and pediatric populations in developed regions.
The blood transfusion segment is expected to witness the fastest growth rate of 26.4% from 2025 to 2032, driven by its widespread use for managing symptomatic anemia across all age groups. Transfusions provide immediate correction of hemoglobin levels, making them a critical supportive therapy alongside emerging targeted treatments. Expanding transfusion services, improved blood safety standards, and growing patient awareness contribute to market growth. Frequent monitoring and integration with iron chelation therapy enhance adoption. The segment is also experiencing growth in emerging regions due to improving hospital infrastructure and accessibility to transfusion services. Its flexibility for both acute and chronic management ensures continued demand.
- By End User
On the basis of end user, the global pyruvate kinase (PK) deficiency market is segmented into hospitals, clinics, research organizations, and others. The hospitals segment dominated with a market share of 56.1% in 2024, due to the availability of comprehensive hematology care, advanced diagnostics, and specialized treatment facilities. Hospitals provide integrated care including transfusions, transplantation, and monitoring of novel therapies. Large hospitals also participate in clinical trials for innovative treatments, increasing adoption of new therapies. The presence of experienced hematologists and multidisciplinary teams supports high-quality patient management. Hospitals in developed regions have higher capacity for rare disease treatment, reinforcing market dominance. Increased hospital-based awareness campaigns also drive early diagnosis and therapy initiation.
The research organizations segment is expected to witness the fastest growth rate of 29.2% from 2025 to 2032, fueled by the expanding focus on drug development, clinical trials, and real-world evidence generation. Research institutes are pivotal in developing next-generation therapies, such as gene editing and enzyme activators. Collaboration between research organizations and pharmaceutical companies accelerates innovation and market readiness of new treatments. Government and private funding for rare disease research is increasing adoption of novel therapies in clinical settings. The segment benefits from advancements in laboratory infrastructure and translational research capabilities. Growing focus on personalized medicine and unmet clinical needs drives faster growth.
- By Distribution Channel
On the basis of distribution channel, the global pyruvate kinase (PK) deficiency market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated with a market share of 47.8% in 2024, owing to direct access to critical therapies, integration with patient care, and professional supervision of high-risk treatments. Hospital pharmacies ensure proper dosing, storage, and administration of complex therapies such as bone marrow transplantation and enzyme activators. They also support monitoring and management of adverse events, increasing patient safety. Strong relationships with hematology departments and clinical trial sites reinforce adoption. Developed countries have high hospital pharmacy penetration, contributing to market dominance. The segment also benefits from healthcare reimbursement structures that prioritize hospital dispensing.
The online pharmacy segment is expected to witness the fastest growth rate of 31.5% from 2025 to 2032, driven by increasing digital healthcare adoption and patient preference for convenient home delivery of medications. Online platforms provide accessibility to supportive treatments such as folic acid, vitamin B, and iron chelation therapy. Expansion of telemedicine services and digital prescription systems is further accelerating adoption. The segment is also growing due to awareness campaigns targeting rare disease patients and caregivers. Increasing penetration of e-pharmacy platforms in emerging markets supports faster growth. Home delivery options enhance adherence to therapy, boosting demand.
Pyruvate Kinase (PK) Deficiency Market Regional Analysis
- North America dominated the global pyruvate kinase (PK) deficiency market with the largest revenue share of 42.8% in 2024, attributed to advanced healthcare infrastructure, high adoption of innovative diagnostics and therapies, strong presence of key pharmaceutical players, and increased investment in rare disease research, particularly in the U.S.
- Patients and healthcare providers in the region highly value access to specialized hematology centers, cutting-edge diagnostics such as genetic testing, and innovative treatment options such as mitapivat and bone marrow transplantation
- This widespread adoption is further supported by strong government initiatives for rare disease management, significant investment in clinical trials, and high healthcare spending, establishing North America as a leading market for PK deficiency therapies in both adult and pediatric populations
U.S. Pyruvate Kinase (PK) Deficiency Market Insight
The U.S. pyruvate kinase (PK) deficiency market captured the largest revenue share of 44% in 2024 within North America, fueled by advanced healthcare infrastructure and the early adoption of novel therapies such as mitapivat and bone marrow transplantation. Patients and healthcare providers are increasingly prioritizing access to specialized hematology centers and comprehensive diagnostic services, including genetic testing and pyruvate kinase activity assays. The growing awareness of rare hematologic disorders and the robust clinical trial ecosystem further propels market growth. In addition, integration of supportive care measures, such as transfusions and iron chelation, complements innovative therapies, enhancing patient outcomes and adoption rates.
Europe Pyruvate Kinase (PK) Deficiency Market Insight
The Europe pyruvate kinase (PK) deficiency market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increased awareness of rare diseases and growing investment in diagnostic and therapeutic development. Early diagnosis through genetic testing and enzyme assays is becoming more prevalent across hospitals and specialty clinics. Rising adoption of advanced treatment options, coupled with government incentives for rare disease management, supports market expansion. The region is also witnessing growth in both pediatric and adult populations, with specialized care centers facilitating access to therapies and improving long-term patient outcomes.
U.K. Pyruvate Kinase (PK) Deficiency Market Insight
The U.K. pyruvate kinase (PK) deficiency market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by heightened awareness of rare hematologic disorders and the increasing availability of innovative treatments. Healthcare providers are adopting key therapies such as bone marrow transplantation and enzyme activators for effective disease management. Furthermore, the growing patient advocacy programs and specialized hematology services are improving diagnosis and treatment access. The country’s robust healthcare system, alongside clinical trial participation, continues to stimulate market growth for both adults and pediatric patients.
Germany Pyruvate Kinase (PK) Deficiency Market Insight
The Germany pyruvate kinase (PK) deficiency market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of rare anemias and the growing demand for advanced diagnostics and therapies. Germany’s well-developed healthcare infrastructure and focus on research and innovation promote adoption of targeted treatments, including mitapivat and hematopoietic stem cell transplantation. Hospitals and specialty clinics are integrating supportive care with advanced therapies for holistic disease management. The emphasis on precision medicine and regulatory support for rare disease drugs further strengthens market growth.
Asia-Pacific Pyruvate Kinase (PK) Deficiency Market Insight
The Asia-Pacific pyruvate kinase (PK) deficiency market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by rising awareness, improving healthcare access, and government initiatives supporting rare disease management in countries such as China, Japan, and India. Expansion of diagnostic capabilities, including genetic testing and enzyme assays, is facilitating early diagnosis. Furthermore, growing adoption of novel therapies, along with increasing investment in pediatric and adult hematology care, is accelerating treatment access. Emerging economies are focusing on rare disease education, patient support programs, and infrastructure development, further propelling market growth.
Japan Pyruvate Kinase (PK) Deficiency Market Insight
The Japan pyruvate kinase (PK) deficiency market is gaining momentum due to high awareness of rare diseases, well-developed healthcare infrastructure, and an increasing focus on personalized medicine. The country emphasizes early diagnosis and comprehensive management of PK deficiency, integrating genetic testing, enzyme assays, and supportive therapies. Growth is further supported by the aging population, which increases demand for specialized hematology care, and by expanding clinical trial participation for innovative therapies. Japan’s technological capabilities also enable better monitoring and management of disease progression.
India Pyruvate Kinase (PK) Deficiency Market Insight
The India pyruvate kinase (PK) deficiency market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to improving healthcare access, expanding specialty care centers, and rising awareness of rare hematologic disorders. The country is witnessing increased adoption of advanced diagnostics such as genetic testing and pyruvate kinase activity assays. Government initiatives promoting rare disease management and growing patient advocacy programs are driving early diagnosis and therapy adoption. The availability of cost-effective supportive treatments, coupled with gradual introduction of novel therapies, further propels market growth in both pediatric and adult populations.
Pyruvate Kinase (PK) Deficiency Market Share
The Pyruvate Kinase (PK) Deficiency industry is primarily led by well-established companies, including:
- Agios Pharmaceuticals, Inc. (U.S.)
- Rocket Pharmaceuticals, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- Ultragenyx Pharmaceutical Inc. (U.S.)
What are the Recent Developments in Global Pyruvate Kinase (PK) Deficiency Market?
- In September 2025, Agios Pharmaceuticals announced that the FDA extended the Prescription Drug User Fee Act (PDUFA) goal date for Mitapivat from September 7, 2025, to December 7, 2025. This extension pertains to the New Drug Application (NDA) for Mitapivat, indicating ongoing regulatory review processes
- In February 2025, Agios Pharmaceuticals reported topline results from the Phase 3 ACTIVATE-KidsT study of Mitapivat in children aged 1 to <18 years with PK deficiency. The study demonstrated that Mitapivat significantly improved hemoglobin levels and reduced hemolysis in pediatric patients, supporting its potential use in younger populations
- In December 2024, Agios Pharmaceuticals presented positive results from the Phase 3 ENERGIZE study, evaluating Mitapivat in adults with non-transfusion-dependent PK deficiency. The study demonstrated significant improvements in hemoglobin levels and reductions in hemolysis, reinforcing the efficacy of Mitapivat as a treatment option for PK deficiency
- In May 2023, Rocket Pharmaceuticals received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA for RP-L301, a lentiviral-based gene therapy for PK deficiency. This designation facilitates the development and potential approval of RP-L301, offering a promising therapeutic avenue for patients with PK deficiency
- In February 2022, the U.S. Food and Drug Administration (FDA) approved Mitapivat (brand name PYRUKYND) as the first disease-modifying treatment for adults with pyruvate kinase (PK) deficiency. This oral medication activates the pyruvate kinase enzyme, improving hemoglobin levels and reducing hemolysis. The approval marked a significant advancement in the management of this rare genetic disorder
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.