Global Rhabdomyosarcoma Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.20 Billion
USD
1.77 Billion
2025
2033
USD
1.20 Billion
USD
1.77 Billion
2025
2033
| 2026 –2033 | |
| USD 1.20 Billion | |
| USD 1.77 Billion | |
|
|
|
|
Rhabdomyosarcoma Treatment Market Size
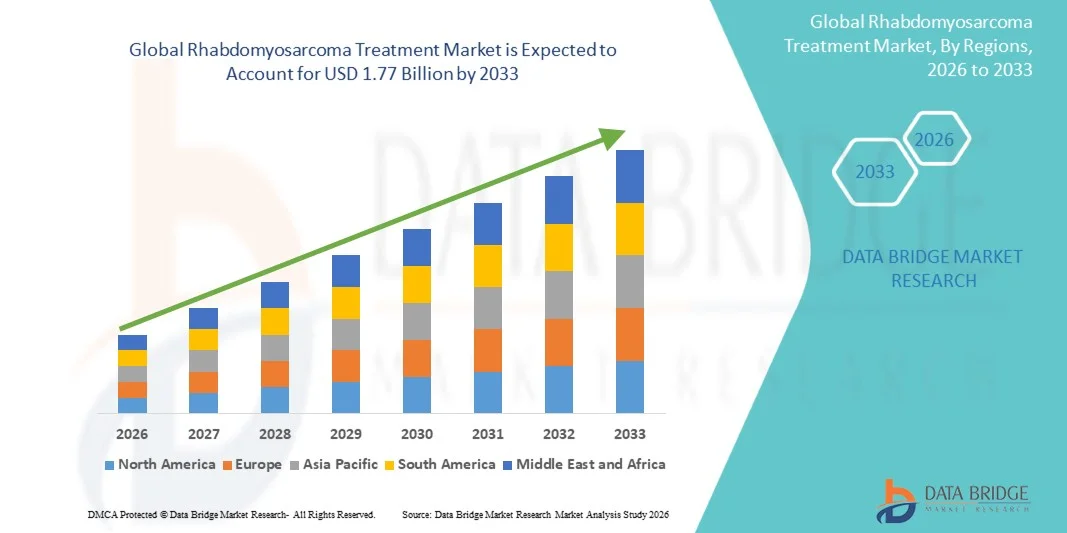
- The global Rhabdomyosarcoma treatment market size was valued at USD 1.20 billion in 2025 and is expected to reach USD 1.77 billion by 2033, at a CAGR of 5.0% during the forecast period
- The market growth is largely fueled by the rising adoption of innovative treatment approaches, including targeted and combination therapies, enhanced clinical research activities, and growing awareness of treatment options among clinicians and caregivers, which are transforming outcomes for patients with this rare soft tissue cancer
- Furthermore, increased investment in oncology R&D, the broader availability of multimodal treatment strategies, and improved healthcare infrastructure worldwide are accelerating the uptake of rhabdomyosarcoma treatments, thereby significantly boosting the industry’s growth as healthcare systems prioritize early diagnosis and improved survival rates for affected populations
Rhabdomyosarcoma Treatment Market Analysis
- Rhabdomyosarcoma treatment, including chemotherapy, targeted therapy, and multimodal approaches, is increasingly vital in improving patient survival and quality of life, due to advancements in precision medicine, early diagnosis, and supportive care that enable more effective and personalized therapeutic strategies
- The escalating demand for Rhabdomyosarcoma treatment is primarily fueled by the rising incidence of pediatric and adult soft tissue sarcomas, increased awareness of treatment options among clinicians, and ongoing clinical research efforts aimed at developing novel therapies with improved efficacy and reduced side effects
- North America dominated the Rhabdomyosarcoma treatment market with the largest revenue share of 38.5% in 2025, driven by advanced healthcare infrastructure, high adoption of innovative therapies, strong oncology R&D investments, and the presence of leading pharmaceutical and biotechnology companies focused on pediatric oncology
- Asia-Pacific is expected to be the fastest-growing region in the Rhabdomyosarcoma treatment market during the forecast period due to rising healthcare expenditure, expanding access to specialized oncology centers, and growing awareness of early diagnosis and treatment options
- Chemotherapy segment dominated the Rhabdomyosarcoma treatment market with a market share of 42.3% in 2025, driven by its established role as a standard of care, broad clinical adoption, and integration into multimodal treatment regimens for effective tumor management
Report Scope and Rhabdomyosarcoma Treatment Market Segmentation
|
Attributes |
Rhabdomyosarcoma Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Rhabdomyosarcoma Treatment Market Trends
“Advancements in Targeted and Immunotherapies”
- A significant and accelerating trend in the global Rhabdomyosarcoma treatment market is the growing adoption of targeted gene therapies and immunotherapies, which are enhancing treatment efficacy while reducing systemic toxicity
- For instance, drugs such as larotrectinib and vincristine-based combination therapies are increasingly utilized in specific rhabdomyosarcoma subtypes, offering more precise and personalized treatment options
- Integration of molecular diagnostics with treatment planning enables oncologists to tailor therapy based on genetic markers, improving response rates and long-term outcomes. For instance, the use of PAX3-FOXO1 fusion gene detection guides therapy selection in high-risk patients
- These advancements are facilitating the shift from conventional chemotherapy toward multimodal approaches, combining surgery, radiation, and targeted therapy for optimized patient outcomes
- This trend toward precision medicine and personalized therapy is reshaping clinical expectations for rhabdomyosarcoma management. Consequently, pharmaceutical companies are increasingly investing in R&D for novel targeted agents and immunotherapies
- The demand for innovative, less toxic, and highly effective Rhabdomyosarcoma Treatment is rising rapidly across pediatric and adult populations, as healthcare systems prioritize improved survival and quality of life
- Another emerging trend is the use of combination immunotherapy with checkpoint inhibitors to enhance anti-tumor response, particularly in refractory or relapsed cases
Rhabdomyosarcoma Treatment Market Dynamics
Driver
“Rising Incidence and Focus on Pediatric Oncology”
- The increasing prevalence of rhabdomyosarcoma, particularly among children and adolescents, coupled with heightened awareness of treatment options, is a significant driver for market growth
- For instance, in 2025, several oncology centers reported a rise in enrollment for clinical trials evaluating novel therapies in pediatric rhabdomyosarcoma patients, reflecting growing therapeutic demand
- As clinicians seek to improve survival rates, Rhabdomyosarcoma Treatment offers advanced regimens, including combination chemotherapy and targeted therapies, providing improved efficacy over traditional treatments
- Furthermore, increasing investments in pediatric oncology research and growing public awareness campaigns are making advanced treatment more accessible, supporting market expansion
- The development of patient-centric care models, integration of multimodal treatment strategies, and access to specialized oncology centers are key factors propelling the adoption of Rhabdomyosarcoma Treatment globally
- Expansion of healthcare infrastructure in emerging markets is providing more children and adults access to specialized oncology centers, supporting market growth
- For instance, new pediatric oncology units in Asia-Pacific and Latin America are increasing treatment availability and improving early diagnosis rates, driving demand for advanced therapies
Restraint/Challenge
“Toxicity Concerns and High Treatment Costs”
- Concerns regarding chemotherapy and targeted therapy side effects pose a significant challenge to broader market adoption, as adverse reactions can limit treatment tolerability
- For instance, reports of severe neutropenia, cardiotoxicity, and growth complications in pediatric patients have made some caregivers hesitant to pursue aggressive therapy regimens
- Addressing these toxicity concerns through safer therapeutic combinations, supportive care, and dose optimization is crucial for improving patient adherence and outcomes. High costs associated with novel targeted and immunotherapies also limit access in developing regions
- For instance, treatment regimens including TRK inhibitors or combination immunotherapies can cost several hundred thousand dollars per patient, creating affordability challenges
- While ongoing research aims to improve safety profiles and reduce costs, these factors remain key hurdles in achieving widespread market penetration, particularly in resource-limited settings
- Regulatory approvals and lengthy clinical trial timelines can delay the availability of innovative therapies, slowing market expansion
- For instance, new immunotherapy candidates may require multiple phases of pediatric trials before approval, limiting early patient access and adoption rates
Rhabdomyosarcoma Treatment Market Scope
The market is segmented on the basis of disease type, treatment, population type, drug type, route of administration, end user, and distribution channel,
- By Disease Type
On the basis of disease type, the market is segmented into Embryonal Rhabdomyosarcoma and Alveolar Rhabdomyosarcoma. The Embryonal Rhabdomyosarcoma segment dominated the market in 2025 with the largest revenue share of 56%, driven by its higher prevalence in children and early detection rates. Embryonal subtypes respond well to multimodal therapy including surgery, chemotherapy, and radiation, making them a significant contributor to overall market revenue. Clinical treatment guidelines often prioritize chemotherapy regimens for embryonal cases, enhancing the uptake of branded and generic drugs. The segment also benefits from robust research and pediatric oncology programs focused on improving survival rates in early-onset patients. Healthcare providers and specialized oncology centers actively manage embryonal rhabdomyosarcoma, ensuring high treatment adoption. In addition, government healthcare initiatives and insurance coverage in developed countries support consistent access to therapy.
The Alveolar Rhabdomyosarcoma segment is expected to witness the fastest growth CAGR 6.2% from 2026–2033, driven by its aggressive nature and increasing awareness of advanced treatment options. Alveolar cases often require targeted therapies and novel immunotherapies, boosting market demand for new drugs. Early detection through molecular diagnostics is increasing treatment uptake in both children and adults. Research pipelines focused on fusion-gene-positive alveolar rhabdomyosarcoma are fueling market expansion. Rising clinical trial activity and growing awareness among oncologists are contributing to faster adoption. Investments in personalized treatment strategies further support revenue growth in this segment.
- By Treatment
On the basis of treatment, the market is segmented into surgery, radiation therapy, chemotherapy, and stem cell therapy. The Chemotherapy segment dominated the market in 2025 with a revenue share of 42.3%, due to its long-standing status as a standard of care across most rhabdomyosarcoma subtypes. Chemotherapy combinations, including vincristine, actinomycin D, and cyclophosphamide, are widely prescribed for both pediatric and adult patients. The segment benefits from broad adoption in hospitals, specialty clinics, and oncology centers globally. It also receives significant support from R&D for novel drug formulations and combination therapies. Availability of both branded and generic chemotherapy drugs ensures high accessibility and steady demand. Clinical guidelines continue to prioritize chemotherapy, supporting consistent revenue generation.
Stem Cell Therapy segment is expected to witness the fastest growth 7.1% CAGR from 2026–2033, driven by its potential in treating relapsed or refractory cases. Stem cell transplantation, often following high-dose chemotherapy, offers improved recovery and long-term survival prospects. Ongoing research into hematopoietic stem cells and regenerative therapy is fueling adoption. The segment is also expanding in emerging markets due to improved healthcare infrastructure. Patient awareness and clinical success stories are increasing physician adoption rates. Integration with personalized medicine further enhances growth potential.
- By Population Type
On the basis of population type, the market is segmented into children and adults. The Children segment dominated the market in 2025 with a revenue share of 61%, as rhabdomyosarcoma is primarily a pediatric cancer. High incidence in children drives demand for early diagnosis, multimodal treatment, and access to specialized pediatric oncology centers. Government initiatives, insurance coverage, and pediatric clinical programs facilitate consistent treatment adoption. Treatment protocols in children often combine surgery, chemotherapy, and radiation, boosting overall therapy consumption. Awareness campaigns for early detection further support the market share. Hospitals and specialty clinics focusing on pediatric oncology prioritize access to advanced drugs and therapies.
The Adults segment is expected to witness the fastest growth 5.8% CAGR from 2026–2033, driven by increasing identification of late-onset rhabdomyosarcoma and the adoption of targeted therapies. Improved diagnostic tools are enabling early detection in adults, increasing treatment initiation. Clinical trials focusing on adult subtypes expand available therapy options. Rising awareness among healthcare professionals and patient advocacy groups fuels adoption. Demand for novel immunotherapies and precision medicine approaches in adults contributes to faster market growth.
- By Drug Type
On the basis of drug type, the market is segmented into branded and generic drugs. The Branded Drugs segment dominated the market in 2025 with a revenue share of 57%, supported by innovative targeted therapies, patent-protected chemotherapy formulations, and established efficacy in clinical trials. Branded drugs often offer enhanced patient support programs and wider insurance coverage. Pharmaceutical companies actively market these drugs through hospitals, specialty clinics, and research institutes, ensuring high uptake. Brand recognition and physician trust contribute to continued dominance. Regulatory approvals in major markets such as the U.S. and Europe further solidify revenue share.
The Generic Drugs segment is expected to witness the fastest growth 6.5% CAGR from 2026–2033, driven by increasing cost-sensitivity and adoption in emerging markets. Generics provide affordable alternatives without compromising efficacy, expanding access to standard chemotherapy regimens. Growing awareness and availability of generics in hospitals and retail pharmacies enhance adoption rates. Initiatives to reduce treatment costs in developing countries further support growth. Volume-based procurement by healthcare providers boosts market penetration.
- By Route of Administration
On the basis of route of administration, the market is segmented into oral and parenteral. The Parenteral segment dominated the market in 2025 with a revenue share of 65%, as most chemotherapy and targeted therapies are administered intravenously for optimal bioavailability. Hospital and specialty clinic settings are equipped to deliver these therapies safely, driving high adoption. Parenteral administration allows precise dosing and combination therapy integration. Physicians prefer parenteral routes for high-risk or aggressive tumors. Clinical guidelines frequently recommend intravenous delivery for pediatric and adult patients. Ongoing R&D into infusion protocols and supportive care improves treatment adherence.
The Oral segment is expected to witness the fastest growth 7.2% CAGR from 2026–2033, driven by the development of oral targeted therapies and kinase inhibitors. Oral drugs enhance patient convenience and compliance, particularly for outpatient care. Improved formulation and bioavailability are expanding adoption in children and adults. Increased physician acceptance and patient preference for at-home treatment accelerate growth. Telemedicine integration supports remote monitoring for oral therapy. Expansion in emerging markets with limited hospital access further fuels market penetration.
- By End User
On the basis of end user, the market is segmented into hospitals, specialty clinics, diagnostic centers, research institutes, and others. The Hospitals segment dominated the market in 2025 with a revenue share of 55%, due to well-established oncology departments, high patient volumes, and access to multimodal therapy options. Hospitals provide comprehensive care, including surgery, chemotherapy, radiation, and supportive therapy. They are preferred for pediatric and high-risk adult patients. Insurance coverage and government programs often support hospital-based treatment. Hospitals also drive adoption of advanced branded drugs and parenteral therapies. Integration with clinical trials further reinforces dominance.
The Specialty Clinics segment is expected to witness the fastest growth 6.8% CAGR from 2026–2033, driven by increasing focus on pediatric oncology, targeted therapy, and outpatient care models. Clinics provide personalized treatment plans and access to newer therapies. For instance, dedicated pediatric oncology clinics are expanding in Asia-Pacific and Latin America. The convenience of specialized care and shorter waiting times attracts patients and physicians. Growing investment in private oncology centers supports this segment’s rapid growth.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, online pharmacies, and others. The Hospital Pharmacy segment dominated the market in 2025 with a revenue share of 58%, as most chemotherapy and parenteral therapies are dispensed directly through hospital channels for controlled administration. Hospitals ensure adherence to dosing protocols, monitor side effects, and provide supportive care, making them a critical distribution channel. Hospitals also facilitate access to branded drugs and clinical trial therapies. The segment benefits from integration with treatment centers and insurance coverage. Physician prescriptions are often fulfilled through hospital pharmacies, enhancing revenue.
The Online Pharmacy segment is expected to witness the fastest growth 9.1% CAGR from 2026–2033, driven by rising digital adoption, patient convenience, and home delivery of oral therapies. Patients increasingly prefer ordering medications online to avoid hospital visits. Telemedicine consultations and e-prescriptions support online pharmacy growth. Expansion in urban and semi-urban regions fuels adoption. Online platforms also provide access to generic drugs at competitive prices, boosting market penetration.
Rhabdomyosarcoma Treatment Market Regional Analysis
- North America dominated the Rhabdomyosarcoma treatment market with the largest revenue share of 38.5% in 2025, driven by advanced healthcare infrastructure, high adoption of innovative therapies, strong oncology R&D investments, and the presence of leading pharmaceutical and biotechnology companies focused on pediatric oncology
- Patients and caregivers in the region highly value access to specialized oncology centers, state-of-the-art treatment modalities, and comprehensive care programs, including surgery, chemotherapy, radiation, and targeted therapies
- This widespread adoption is further supported by significant R&D investment from pharmaceutical companies, insurance coverage for cancer therapies, and increasing enrollment in clinical trials, establishing North America as a key hub for rhabdomyosarcoma treatment and innovation
U.S. Rhabdomyosarcoma Treatment Market Insight
The U.S. Rhabdomyosarcoma treatment market captured the largest revenue share of 82% in 2025 within North America, driven by advanced pediatric oncology centers, high adoption of multimodal therapies, and extensive clinical research programs. Patients and caregivers increasingly prioritize access to state-of-the-art treatment options, including chemotherapy, targeted therapy, and stem cell transplantation. The growing enrollment in clinical trials for novel immunotherapies and personalized medicine is further propelling market growth. Moreover, strong insurance coverage, early detection programs, and widespread awareness among healthcare providers significantly contribute to treatment accessibility. The integration of multidisciplinary care teams ensures improved survival outcomes, reinforcing the dominance of the U.S. market.
Europe Rhabdomyosarcoma Treatment Market Insight
The Europe Rhabdomyosarcoma treatment market is projected to expand at a substantial CAGR during the forecast period, primarily driven by well-established healthcare systems, early diagnosis programs, and accessibility to advanced therapies. Rising urbanization and the adoption of standardized pediatric oncology treatment protocols foster market growth. European clinicians emphasize multimodal treatment strategies, combining surgery, chemotherapy, and radiation, to improve patient outcomes. Government initiatives, research programs, and cross-border collaborations in oncology further support market expansion. The region is witnessing growth across both pediatric and adult populations, with increased uptake in hospitals and specialty clinics.
U.K. Rhabdomyosarcoma Treatment Market Insight
The U.K. Rhabdomyosarcoma treatment market is anticipated to grow at a noteworthy CAGR, driven by increasing awareness of pediatric cancers and demand for advanced therapeutic options. Rising concerns regarding survival outcomes and long-term quality of life are encouraging early treatment intervention. The U.K.’s well-developed healthcare infrastructure and integration of oncology specialists into treatment planning further support adoption. In addition, government-funded programs, research initiatives, and robust clinical trial participation are fueling market growth. Access to targeted therapies and patient-centric care models reinforces the country’s position as a key market within Europe.
Germany Rhabdomyosarcoma Treatment Market Insight
The Germany Rhabdomyosarcoma treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by advanced medical infrastructure and emphasis on high-quality oncology care. Awareness of pediatric and adult rhabdomyosarcoma management among healthcare providers is driving adoption of multimodal therapies. Germany’s focus on innovation and access to novel targeted therapies supports the market growth. Hospitals and specialty clinics are equipped with state-of-the-art surgical and radiation facilities, ensuring optimal patient outcomes. Moreover, integration of clinical research programs and participation in international oncology trials enhances treatment availability. Growing emphasis on patient safety and long-term follow-up care further contributes to the market expansion.
Asia-Pacific Rhabdomyosarcoma Treatment Market Insight
The Asia-Pacific Rhabdomyosarcoma treatment market is poised to grow at the fastest CAGR of 6.5% during 2026–2033, driven by increasing healthcare expenditure, expanding pediatric oncology infrastructure, and rising awareness of advanced treatment options. The region’s focus on early diagnosis and access to standardized multimodal therapies is boosting adoption. Government initiatives, private oncology clinics, and collaborations with international pharmaceutical companies are facilitating market growth. Improved affordability of generic chemotherapy and expansion of specialized care centers are further supporting adoption. Countries such as China, India, and Japan are leading growth, with increasing enrollment in clinical trials and telemedicine adoption enhancing access to treatment.
Japan Rhabdomyosarcoma Treatment Market Insight
The Japan Rhabdomyosarcoma treatment market is gaining momentum due to the country’s high healthcare standards, advanced pediatric oncology programs, and emphasis on innovative therapies. Rapid urbanization and the integration of precision medicine into clinical practice are increasing treatment uptake. Patients benefit from access to multimodal therapy regimens, including surgery, chemotherapy, targeted therapy, and immunotherapy. Rising awareness among caregivers and healthcare professionals further supports early diagnosis and intervention. Japan’s aging population also drives demand for tailored treatment approaches in adult patients. In addition, participation in international clinical trials ensures availability of cutting-edge therapies in the region.
India Rhabdomyosarcoma Treatment Market Insight
The India Rhabdomyosarcoma treatment market accounted for the largest revenue share in Asia-Pacific in 2025, attributed to expanding pediatric healthcare infrastructure, increasing urbanization, and rising awareness of cancer treatment options. India has become a key market for access to both branded and generic chemotherapy drugs. Government programs, private oncology centers, and telemedicine initiatives are enhancing treatment accessibility. Growing enrollment in clinical trials and collaboration with global pharmaceutical companies are accelerating adoption of novel therapies. The availability of affordable oral and parenteral therapies, alongside specialized pediatric oncology care, is a major factor propelling market growth.
Rhabdomyosarcoma Treatment Market Share
The Rhabdomyosarcoma Treatment industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- F. Hoffmann‑La Roche Ltd (Switzerland)
- Novartis AG (Switzerland)
- Bristol‑Myers Squibb Company (U.S.)
- Merck & Co., Inc. (U.S.)
- Eli Lilly and Company (U.S.)
- AstraZeneca U.K.)
- Sanofi (France)
- GSK plc (U.K.)
- Takeda Pharmaceutical Company Limited (Japan)
- Bayer AG (Germany)
- AbbVie Inc. (U.S.)
- Amgen Inc. (U.S.)
- Ipsen (France)
- BeOne Medicines (U.S.)
- Eisai Co., Ltd (Japan)
- Gilead Sciences, Inc. (U.S.)
- Exelixis, Inc. (U.S.)
- Edison Oncology (U.S.)
What are the Recent Developments in Global Rhabdomyosarcoma Treatment Market?
- In October 2025, new research from Fred Hutchinson Cancer Center highlighted discoveries around how therapy drives molecular evolution in relapsed rhabdomyosarcoma, identifying gene fusion mechanisms that help tumors resist treatment and pointing toward future targeted therapy strategies that could improve relapse management and precision‑based care
- In March 2025, long‑term outcome data from the European Paediatric Soft Tissue Sarcoma Study Group (RMS2005 trial) showed that incorporating maintenance chemotherapy (vinorelbine + low‑dose cyclophosphamide) significantly improved 10‑year overall survival and event‑free survival in high‑risk rhabdomyosarcoma patients, reinforcing the value of extended chemotherapy strategies in clinical practice and influencing treatment protocols
- In October 2023, Oncoheroes Biosciences announced that the U.S. FDA allowed the company to proceed with two pediatric oncology clinical trials, including one for volasertib a potential rhabdomyosarcoma treatment after review of its Investigational New Drug (IND) applications. This regulatory clearance opened the door to clinical evaluation of new therapeutic options for children with aggressive tumors such as rhabdomyosarcoma
- In September 2023, a scientific study published in Cell Reports Medicine described a breakthrough CAR‑T cell therapy for RMS in mice, significantly reducing tumor burden and supporting the upcoming transition into potential Phase I clinical trials aimed at patients with aggressive rhabdomyosarcoma unresponsive to conventional multimodal treatment
- In September 2023, Oncoheroes Biosciences also received FDA Rare Pediatric Disease Designation (RPDD) and Orphan Drug incentives for volasertib’s use in rhabdomyosarcoma, accelerating development pathways by facilitating priority review vouchers and regulatory incentives that can speed future approval processes
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.