Global Risuteganib In Neurological Disorder Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
2.27 Billion
USD
3.30 Billion
2024
2032
USD
2.27 Billion
USD
3.30 Billion
2024
2032
| 2025 –2032 | |
| USD 2.27 Billion | |
| USD 3.30 Billion | |
|
|
|
|
Risuteganib in Neurological Disorder Treatment Market Size
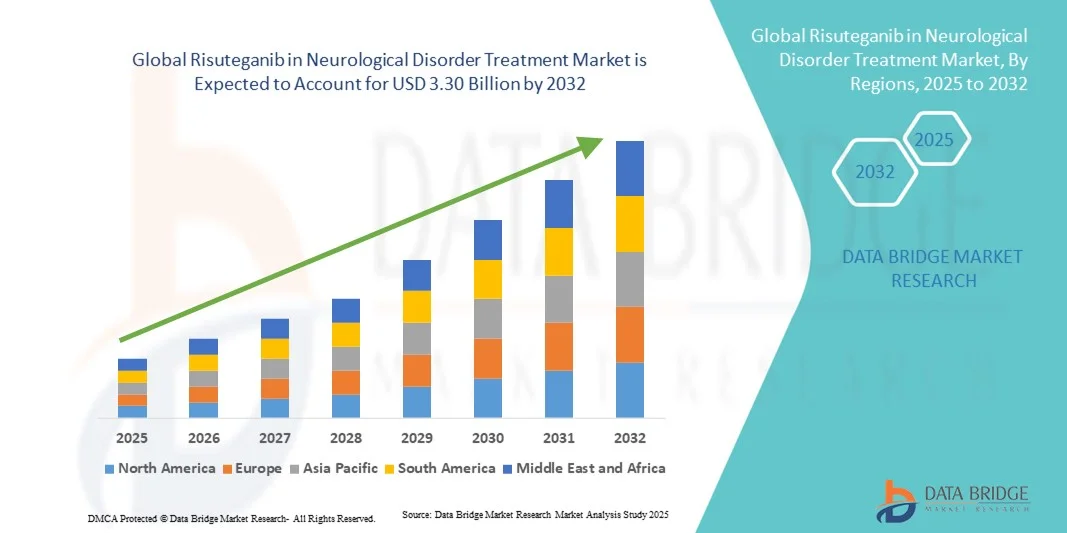
- The global Risuteganib in Neurological Disorder Treatment market size was valued at USD 2.27 billion in 2024 and is expected to reach USD 3.30 billion by 2032, at a CAGR of 4.80% during the forecast period
- The market growth is largely fuelled by the growing prevalence of neurological disorders and increasing interest in innovative treatment modalities, including novel therapeutics such as Risuteganib, which is being explored beyond its original ophthalmic indications
- Furthermore, rising demand for more effective, targeted therapies in conditions such as Alzheimer’s disease, Parkinson’s disease, multiple sclerosis and other neuro‑degenerative disorders is establishing treatments such as Risuteganib as potential modern options in the access control of neurological disease management
Risuteganib in Neurological Disorder Treatment Market Analysis
- Risuteganib, a novel therapeutic targeting integrin and complement pathways, is increasingly explored for the treatment of neurological disorders such as Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative conditions due to its potential to modify underlying disease mechanisms rather than only alleviating symptoms
- The escalating demand for Risuteganib in Neurological Disorder Treatment is primarily fueled by the growing prevalence of neurodegenerative and age-related disorders, an aging global population, stronger R&D investment in precision neurology, and increasing awareness of therapies targeting complement and integrin systems in neurological conditions
- North America dominated the Risuteganib in Neurological Disorder Treatment market with the largest revenue share of 47.9% in 2024, characterized by advanced healthcare infrastructure, early adoption of novel neurology therapeutics, strong pharmaceutical R&D presence, and a favourable regulatory environment, with the U.S. leading in clinical trials, drug approvals, and adoption of Risuteganib-based treatments
- Asia-Pacific is expected to be the fastest-growing region in the Risuteganib in Neurological Disorder Treatment market during the forecast period due to rapidly aging populations, growing healthcare access and spending, increasing diagnosis rates of neurological disorders, and rising awareness of advanced therapeutics
- Neuroprotectants segment dominated the Risuteganib in Neurological Disorder Treatment market with a market share of 43% in 2024, driven by the high unmet medical need for disease-modifying therapies and the growing potential of Risuteganib to provide targeted benefits in neurodegenerative disorder management
Report Scope and Risuteganib in Neurological Disorder Treatment Market Segmentation
|
Attributes |
Risuteganib in Neurological Disorder Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Risuteganib in Neurological Disorder Treatment Market Trends
Targeted Complement‑Cascade Modulation for Neurodegenerative Disorders
- A significant and accelerating trend in the global Risuteganib in Neurological Disorder Treatment market is the deepening focus on complement system (C3/C5) and integrin pathways as disease‑modifying targets rather than only providing symptomatic relief
- For instance, risuteganib’s mechanism of action involves inhibition of complement‑mediated neuronal or retinal damage, offering potential for slowing progression of neurodegeneration in conditions beyond ophthalmic indications
- This trend is driving development of both intravitreal and systemic formulations of risuteganib and its analogues, enabling treatment paradigms that integrate precision medicine and biomarker‑guided patient selection
- The seamless integration of risuteganib into broader neurotherapeutic pipelines and combination therapies is reshaping treatment expectations for neuro‑ophthalmic and neurodegenerative disorders asuch as
- The demand for therapies that offer targeted, mechanism‑based intervention over conventional broad‑spectrum treatments is growing rapidly across both ophthalmology and neurology sectors, as patients and physicians prioritise interventions with deeper biological impact
- For instance, ongoing clinical trials are increasingly using imaging biomarkers and genetic profiling to optimise patient selection, improving efficacy outcomes and accelerating adoption
- Advances in formulation technologies, including sustained-release and ocular delivery systems, are enhancing therapeutic potential and patient adherence, further driving this trend
Risuteganib in Neurological Disorder Treatment Market Dynamics
Driver
Escalating Prevalence of Neurodegenerative and Age‑Related Disorders
- The increasing prevalence of neurodegenerative and age‑related disorders, including those with retinal/neurological overlap, is a primary driver for the risuteganib market
- For instance, global ageing trends and rising incidence of conditions such as dry age‑related macular degeneration and other neurological disorders are expanding the eligible patient pool for risuteganib‑type therapies
- Growing awareness of the unmet medical need for treatments that modify disease progression rather than just alleviate symptoms is reinforcing this driver, increasing investment and clinical trial activity
- Healthcare systems globally are recognising the long‑term cost and burden of neurodegenerative conditions, which incentivises adoption of innovative therapies such as risuteganib that aim to slow or prevent progression
- The confluence of demographic shifts, epidemiological burden, and expanded diagnostic capabilities across regions is making risuteganib’s growth trajectory stronger. For instance, partnerships between biotech companies and academic institutions are accelerating translational research, providing new insights into therapeutic applications for neurological disorders
- Rising government and private funding initiatives supporting neurodegenerative disease research are enhancing pipeline development, boosting growth potential for risuteganib-based treatments
Restraint/Challenge
High Development Cost and Regulatory Complexity
- The high development and manufacturing costs associated with risuteganib, especially given its biologic/advanced therapeutic nature, represent a significant restraint on market growth
- For instance, conducting long‑duration clinical trials for neurodegenerative disorders, complying with complex regulatory requirements for complement‑modulating agents, and managing cold‑chain logistics all contribute to elevated cost and risk
- Regulatory complexity and safety monitoring requirements for novel mechanisms such as complement inhibition present additional hurdles to faster market access
- The presence of established therapies and high unmet expectations in ophthalmology raise the bar for risuteganib’s clinical efficacy and cost‑effectiveness, which may slow reimbursement and adoption
- Ensuring global market access, patient adherence, and equitable pricing across emerging regions adds further complexity to risuteganib’s commercial roll‑out
- For instance, stringent reporting and post-marketing surveillance requirements increase operational burden for manufacturers, delaying market entry in multiple regions
- Variability in regulatory approvals across countries and regions may fragment the market, complicating global launch strategies and scaling efforts for risuteganib
Risuteganib in Neurological Disorder Treatment Market Scope
The market is segmented on the basis of type, application, end-users, and distribution channel.
- By Type
On the basis of type, the Risuteganib in Neurological Disorder Treatment market is segmented into angiostatic proteins, anti-inflammatories, carboxylic acids, eye disorder therapies, neuroprotectants, oligopeptides, pyrrolidines, and small molecules. The neuroprotectants segment dominated the Risuteganib in Neurological Disorder Treatment market with a market share of 43% in 2024, driven by the increasing focus on disease-modifying therapies for neurodegenerative disorders. Neuroprotectants are gaining traction due to rising prevalence of conditions such as Alzheimer’s and Parkinson’s disease. For instance, risuteganib’s neuroprotective mechanism targeting complement and integrin pathways is generating strong clinical and commercial interest. Increased investment in translational research and biomarker-guided therapies is accelerating adoption. Patients and clinicians are increasingly seeking treatments that not only manage symptoms but slow disease progression. Emerging neuroprotectant therapies are capturing attention for their potential in overlapping ophthalmic and neurological disorders.
The small molecules segment is anticipated to witness the fastest growth from 2025 to 2032, fueled by their ease of synthesis, systemic delivery options, and lower development complexity compared to biologics or peptides. For instance, small molecules allow intravitreal and systemic administration, making clinical adoption easier. They also benefit from more predictable regulatory pathways and established manufacturing processes. Physicians often prioritize small molecules due to their broad application potential and proven safety profiles. Ongoing clinical trials and pipeline development continue to focus heavily on small molecule candidates. The rising demand for small molecules in neurodegenerative and ophthalmic indications supports their rapid growth trajectory.
- By Application
On the basis of application, the market is segmented into dry age-related macular degeneration (AMD), retinal disorders, wet AMD, neurological disorder, and others. The dry age-related macular degeneration (AMD) segment dominated the market in 2024 due to the large patient population and lack of effective approved therapies. Physicians prioritize early intervention in dry AMD to prevent progression to vision loss. For instance, risuteganib’s mechanism targeting integrin pathways aligns closely with dry AMD pathology, driving adoption. The segment benefits from established clinical trials and strong R&D investment. Payors and healthcare systems support treatments that prevent costly progression to wet AMD, reinforcing dominance. The segment also receives robust funding and research attention, maintaining its leading market position.
The neurological disorder segment is expected to witness the fastest growth during the forecast period as risuteganib extends its therapeutic potential beyond ophthalmology. Rising prevalence of neurodegenerative disorders and aging populations drive demand. For instance, risuteganib’s modulation of complement and integrin pathways addresses unmet needs in neuroprotection. Increasing clinical trial activity and research partnerships are accelerating development. Patients and clinicians are showing growing interest in disease-modifying neurological therapies. The segment represents high growth potential due to unmet medical needs and novel mechanisms of action.
- By End-Users
On the basis of end-users, the market is segmented into clinic, hospital, and others. The hospital segment dominated the market due to its capability to administer advanced therapies such as intravitreal injections and complex neurological interventions. Hospitals have established infrastructure for patient monitoring and specialist care. For instance, risuteganib administration requires trained healthcare professionals and access to advanced clinical facilities. Hospitals also manage reimbursement pathways and long-term patient follow-up, reinforcing their dominant position. High patient volumes and multidisciplinary teams contribute to adoption. Hospitals remain the preferred setting for controlled therapy delivery and regulatory compliance.
The clinic segment is anticipated to witness the fastest growth from 2025 to 2032, driven by the expansion of outpatient care models for specialty therapies. Clinics offer convenience, lower cost settings, and increased patient preference. For instance, ophthalmology and neurology clinics are adopting risuteganib for localized therapy delivery. Technological advances in patient monitoring enable safe administration outside hospitals. Growing trust in specialty clinics and the trend toward home-based care are accelerating adoption. Clinics are becoming more equipped to handle complex therapies efficiently, driving faster growth.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market in 2024 due to its role in controlled distribution of advanced therapies requiring cold-chain management and clinician administration. Hospitals ensure proper storage, dosing, and patient monitoring. For instance, risuteganib administration involves strict handling protocols, making hospital pharmacies critical. This segment also benefits from established reimbursement networks and clinician trust. Hospital pharmacies maintain regulatory compliance and provide high-volume delivery capabilities. They remain the primary channel for specialist therapies.
The online pharmacy segment is expected to witness the fastest growth during the forecast period as digital health platforms and home delivery for specialty drugs expand globally. For instance, emerging telehealth and e-pharmacy models enable patients to receive therapies at home under supervision. Online pharmacies increase patient convenience and reduce treatment barriers. Digital prescription management supports adherence and monitoring. Growth is also fueled by healthcare digitization and increasing consumer comfort with online therapy procurement. Online channels are gaining traction for novel therapies with remote monitoring support.
Risuteganib in Neurological Disorder Treatment Market Regional Analysis
- North America dominated the Risuteganib in Neurological Disorder Treatment market with the largest revenue share of 47.9% in 2024, characterized by advanced healthcare infrastructure, early adoption of novel neurology therapeutics, strong pharmaceutical R&D presence, and a favourable regulatory environment
- Patients and physicians in the region highly value the targeted mechanism of action, disease-modifying potential, and clinical trial-backed efficacy offered by risuteganib in both ophthalmic and neurological indications
- This widespread adoption is further supported by high healthcare spending, advanced diagnostic capabilities, and strong regulatory support for novel therapies, establishing risuteganib as a preferred treatment solution in hospitals and specialty clinics
U.S. Risuteganib in Neurological Disorder Treatment Market Insight
The U.S. Risuteganib in Neurological Disorder Treatment market captured the largest revenue share of 81% in 2024 within North America, fueled by the high prevalence of neurodegenerative and retinal disorders and advanced healthcare infrastructure. Patients and clinicians increasingly prioritize targeted, disease-modifying therapies such as risuteganib for dry AMD and neurological indications. The growing number of clinical trials and research partnerships, along with strong insurance coverage and reimbursement policies, further propels the market. Moreover, the widespread adoption of outpatient specialty clinics and hospital-based therapy centers is significantly contributing to the market's expansion. High patient awareness, physician preference for novel mechanisms of action, and robust pharmaceutical R&D support strengthen market penetration.
Europe Risuteganib in Neurological Disorder Treatment Market Insight
The Europe market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising incidence of retinal and neurodegenerative disorders and increasing adoption of innovative therapies. Countries such as Germany, France, and Italy are investing in specialty neurology and ophthalmology centers, supporting early risuteganib adoption. The market growth is further encouraged by increasing government healthcare initiatives, awareness programs, and funding for neurodegenerative disease research. European patients are also drawn to therapies offering disease modification rather than only symptomatic relief. The region is experiencing significant uptake in hospitals and clinics, with risuteganib being incorporated into both treatment protocols and clinical trial settings.
U.K. Risuteganib in Neurological Disorder Treatment Market Insight
The U.K. market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing demand for advanced neuroprotective and ophthalmic treatments. Rising prevalence of dry AMD, neurological disorders, and overlap conditions is motivating healthcare providers to adopt innovative therapies such as risuteganib. In addition, strong healthcare infrastructure, government support for clinical research, and patient awareness regarding early intervention are expected to stimulate market growth. The country’s advanced diagnostic capabilities and adoption of specialized treatment centers facilitate faster uptake of novel therapies. The increasing interest in precision medicine approaches further encourages adoption in both hospital and clinic settings.
Germany Risuteganib in Neurological Disorder Treatment Market Insight
The Germany market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s focus on advanced healthcare, research-driven adoption, and awareness of neurodegenerative and ophthalmic conditions. Germany’s well-developed infrastructure, strong pharmaceutical industry, and emphasis on innovative treatments promote adoption of risuteganib. For instance, increasing clinical trial activity in dry AMD and neurodegenerative disorders is accelerating market growth. The integration of therapies into specialty hospitals and outpatient clinics enhances accessibility. Patients and clinicians show preference for disease-modifying therapies, while supportive reimbursement policies reinforce market expansion.
Asia-Pacific Risuteganib in Neurological Disorder Treatment Market Insight
The Asia-Pacific market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by increasing prevalence of retinal and neurological disorders, rising healthcare access, and improved diagnostic capabilities in countries such as China, Japan, and India. Growing awareness of novel therapies and government initiatives promoting advanced treatment options are accelerating adoption. For instance, the expansion of specialty hospitals and outpatient clinics enhances patient access to risuteganib. In addition, increasing research collaborations and funding for neurodegenerative disease management are driving market growth. The region’s focus on precision medicine and early intervention is supporting adoption in both urban and semi-urban areas.
Japan Risuteganib in Neurological Disorder Treatment Market Insight
The Japan market is gaining momentum due to high prevalence of age-related retinal and neurological disorders, advanced healthcare infrastructure, and strong patient awareness. The increasing number of specialty ophthalmology and neurology clinics enables wider access to risuteganib treatments. For instance, clinical studies and real-world evidence supporting neuroprotective benefits of risuteganib are driving physician adoption. Integration of novel therapies into hospital and outpatient care models is enhancing patient compliance. Japan’s aging population and high healthcare expenditure contribute to increased demand for disease-modifying treatments. Furthermore, regulatory support and robust R&D investment in neurodegenerative therapies are boosting market growth.
India Risuteganib in Neurological Disorder Treatment Market Insight
The India market accounted for a significant market revenue share in Asia-Pacific in 2024, attributed to increasing prevalence of retinal disorders and growing awareness of neurological diseases. Expanding specialty healthcare centers, government initiatives promoting advanced treatments, and rising patient affordability support adoption. For instance, urban hospitals and ophthalmology clinics are increasingly offering risuteganib as part of advanced treatment protocols. The country’s expanding middle class and increasing disposable income further drive demand. Availability of clinical trials, partnerships with global pharma companies, and growing physician awareness are key factors propelling market growth. In addition, India’s focus on modernizing healthcare infrastructure accelerates access to novel therapies in both hospital and clinic settings.
Risuteganib in Neurological Disorder Treatment Market Share
The Risuteganib in Neurological Disorder Treatment industry is primarily led by well-established companies, including:
- Allegro Ophthalmics, LLC. (U.S.)
- Hanmi Pharm.Co.,Ltd. (South Korea)
- AffaMed Therapeutics. (China)
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- Johnson & Johnson Services, Inc. (U.S.)
- F. Hoffmann-La Roche AG (Switzerland)
- Amgen Inc. (U.S.)
- AstraZeneca (U.K.)
- Eli Lilly and Company (U.S.)
- Bayer AG (Germany)
- Biogen Inc. (U.S.)
- GSK plc (U.K.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- UCB S.A. (Belgium)
- Lundbeck A/S (Denmark)
- Mitsubishi Tanabe Pharma Corporation (Japan)
- Vertex Pharmaceuticals Incorporated (U.S.)
- Sandoz Group AG (Germany)
What are the Recent Developments in Global Risuteganib in Neurological Disorder Treatment Market?
- In December 2023, Allegro Ophthalmics received approval from the FDA for the design of its Phase 2b/3 clinical trial of risuteganib in intermediate dry AMD, confirming acceptable protocol elements (dose selection, endpoints, analyses) under the SPA agreement.
- In September 2023, AffaMed Therapeutics announced that China’s National Medical Products Administration (NMPA) approved its Clinical Trial Application (CTA) for risuteganib (Luminate®) for the treatment of intermediate dry age‑related macular degeneration (AMD) in Mainland China
- In April 2023, Allegro Ophthalmics secured approval from the U.S. Food & Drug Administration (FDA) via a Special Protocol Assessment (SPA) for the design of its Phase 2b/3 clinical trial of risuteganib (Luminate / ALG‑1001) for intermediate, non‑exudative age‑related macular degeneration
- In April 2022, Allegro Ophthalmics, LLC announced that it would present new scientific data on Risuteganib at the 2022 Association for Research in Vision and Ophthalmology (ARVO) annual meeting focused on its mechanism of action (oxidative stress/mitochondrial pathways) in retinal pigment epithelium cells
- In August 2021, The results of a U.S. Phase 2a trial for Risuteganib in intermediate non‑exudative (dry) Age‑related Macular Degeneration (AMD) were published, showing that 48 % of patients in the Risuteganib arm achieved a gain of ≥ 8 ETDRS letters vs 7 % in the sham arm (p=0.013). The study found no drug‑related serious adverse events
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.