Global Sanfilippo A Market
Market Size in USD Million
CAGR :
% 
 USD
11.64 Million
USD
23.70 Million
2024
2032
USD
11.64 Million
USD
23.70 Million
2024
2032
| 2025 –2032 | |
| USD 11.64 Million | |
| USD 23.70 Million | |
|
|
|
|
Sanfilippo A Market Size
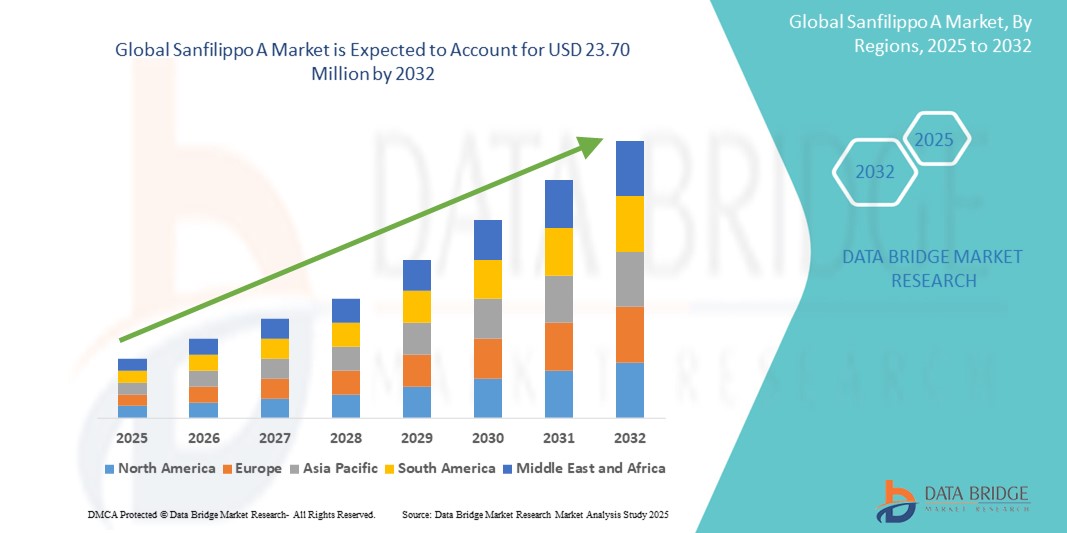
- The global Sanfilippo A market size was valued at USD 11.64 Million in 2024 and is expected to reach USD 23.70 Million by 2032, at a CAGR of 9.30% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within rare disease diagnostics and gene therapy platforms, leading to increased innovation in both research and clinical treatment settings
- Furthermore, rising global demand for targeted, patient-centric, and effective therapies for neurodegenerative lysosomal storage disorders is establishing Sanfilippo A solutions as a vital segment within the rare disease treatment landscape. These converging factors are accelerating the uptake of Sanfilippo A solutions, thereby significantly boosting the industry's growth
Sanfilippo A Market Analysis
- Sanfilippo A, a rare lysosomal storage disorder caused by a deficiency in the enzyme heparan N-sulfamidase, is increasingly gaining attention in the rare disease therapeutics landscape due to the progressive and degenerative nature of the condition, which affects neurological function in children and leads to severe cognitive decline, behavioral issues, and shortened life expectancy
- The growing demand for effective Sanfilippo A treatments is primarily driven by increasing awareness among healthcare providers, the expansion of rare disease research funding, and the acceleration of gene and enzyme replacement therapy development by biotech firms targeting unmet medical needs
- North America dominated the Sanfilippo A market with the largest revenue share of 41.6% in 2024, characterized by early access to clinical trials, favorable reimbursement structures, and the strong presence of research institutions and pharmaceutical companies focused on rare pediatric disorders. The U.S. is witnessing significant growth in treatment access and diagnosis rates, supported by regulatory incentives such as orphan drug designations and expedited approval pathways
- Asia-Pacific is expected to be the fastest growing region in the Sanfilippo A market during the forecast period, with a projected CAGR of 19.4% from 2025 to 2032, due to increasing awareness, expanding healthcare infrastructure, and rising investments in rare disease research by regional governments and global collaborators
- Mucopolysaccharidosis Type III (Sanfilippo Syndrome) segment dominated the Sanfilippo A market with a market share of 42.7% in 2024, driven by targeted research efforts, higher diagnostic awareness, and a growing patient focus. This segment benefits from increased clinical trial activity and disease-specific foundations supporting early detection and therapeutic development
Report Scope and Sanfilippo A Market Segmentation
|
Attributes |
Sanfilippo A Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Sanfilippo A Market Trends
“Emergence of Innovative Therapeutic Strategies and Early Intervention Models”
- A significant and accelerating trend in the Sanfilippo A market is the shift toward innovative therapeutic approaches, including gene therapy, enzyme replacement therapy (ERT), and substrate reduction therapy, aimed at addressing the underlying causes of the disease rather than just managing symptoms. These advancements are reshaping treatment expectations and patient outcomes
- For instance, several companies such as REGENXBIO, Abeona Therapeutics, and Lysogene are actively advancing gene therapy candidates in clinical trials targeting the SGSH gene mutation responsible for Sanfilippo A. These therapies are designed to restore enzymatic function and potentially halt or reverse neurological degeneration
- In parallel, developments in intrathecal and intracerebroventricular delivery methods are improving central nervous system (CNS) drug targeting, which is critical given the neurological nature of the disease. These delivery advancements enhance the potential efficacy of therapies by improving bioavailability in the brain
- The integration of newborn screening programs in certain countries is enabling early diagnosis, allowing for pre-symptomatic intervention which can significantly delay the onset and progression of symptoms. This model of early detection and early treatment is gaining traction globally, with governments and healthcare systems expanding rare disease screening initiatives
- Moreover, patient advocacy organizations are playing a crucial role in driving awareness, funding research, and improving access to clinical trials. These collaborations between stakeholders are contributing to a more connected ecosystem, where research, diagnostics, and treatment are increasingly aligned for more effective care delivery
- This trend toward targeted, early, and disease-modifying interventions is fundamentally transforming the Sanfilippo A landscape, shifting it from symptom management to long-term therapeutic solutions. As regulatory support for rare diseases continues to grow and more clinical data becomes available, the market is expected to experience sustained innovation and growth
Sanfilippo A Market Dynamics
Driver
“Growing Need Due to Rising Diagnostic Awareness and Orphan Drug Development”
- The increasing prevalence of rare genetic disorders like Sanfilippo A, coupled with rising awareness among healthcare professionals and families, is a significant driver for the heightened demand for early diagnosis, therapeutic research, and supportive care solutions in the global Sanfilippo A market
- For instance, in April 2024, Abeona Therapeutics announced progress in its gene therapy program for Sanfilippo syndrome type A (MPS IIIA), highlighting positive outcomes in long-term follow-up studies. Such advancements and strategic initiatives by key biotech firms are expected to drive the Sanfilippo A market growth in the forecast period
- As more families and clinicians recognize the symptoms of Sanfilippo A early—such as developmental delays, speech loss, and behavioral changes—the demand for genetic testing, enzyme assays, and clinical interventions continues to rise. Early diagnosis enables timely supportive care and potential enrollment in clinical trials
- Furthermore, growing support from rare disease foundations, patient advocacy organizations, and international collaborations is pushing research momentum, particularly for gene therapy and enzyme replacement therapies targeting neurological symptoms associated with Sanfilippo A
- The increasing emphasis on newborn screening programs and advancements in molecular diagnostics are key factors propelling the detection and management of Sanfilippo A across pediatric healthcare settings. Government incentives and regulatory frameworks like the Orphan Drug Act and Rare Pediatric Disease Priority Review Voucher are also encouraging biotech innovation and investment in this space
Restraint/Challenge
“Challenges in Therapy Development and High Treatment Costs”
- The lack of curative treatment and the complexity of developing central nervous system-targeted therapies pose a significant challenge to broader clinical success. Sanfilippo A primarily affects the brain, and delivering therapeutic agents across the blood-brain barrier remains a major scientific hurdle
- For instance, several past clinical trials have faced limitations in demonstrating long-term neurological improvement, which has created hesitation in market uptake and funding continuity for some experimental programs
- Addressing these challenges requires sustained investment in advanced drug delivery systems, robust clinical trial designs, and deeper understanding of disease progression. Companies such as Lysogene and Orchard Therapeutics are actively working to overcome these obstacles through novel AAV-based gene therapy platforms
- In addition, the high cost of treatment and supportive care—ranging from diagnostics to long-term multidisciplinary care—can be a barrier to access, especially in low- and middle-income regions. While orphan drug incentives help reduce some development risks, the affordability and reimbursement of eventual therapies remain a concern
- While pricing frameworks and patient access programs are gradually improving, the financial burden for families and health systems continues to limit widespread adoption of available and future treatments
- Overcoming these barriers through public-private partnerships, rare disease funding initiatives, and global access strategies will be vital for sustained growth in the Sanfilippo A market
Sanfilippo A Market Scope
The market is segmented on the basis of diagnosis, disease type, treatment, mode of administration, and end user.
• By Diagnosis
On the basis of diagnosis, the Sanfilippo A market is segmented into GAG analysis, activity assay, genomic DNA sequencing, and others. The GAG analysis segment dominated with the largest revenue share of 36.4% in 2024, owing to its widespread use as a primary biochemical screening tool.
The genomic DNA sequencing segment is expected to grow at the fastest CAGR of 18.2% from 2025 to 2032, due to increased adoption of genetic diagnostics and personalized medicine.
• By Disease Type
On the basis of disease type, the Sanfilippo A market is segmented into Mucopolysaccharidosis Type I, Type II, Type III, Type IV A, Type VI, and others. Mucopolysaccharidosis Type III (Sanfilippo Syndrome) held the largest share of 42.7% in 2024, driven by targeted research and a high patient focus.
MPS Type II is projected to grow at a fastest CAGR of 16.9% during 2025–2032, supported by emerging therapies and expanding diagnosis programs.
• By Treatment
On the basis of treatment, the Sanfilippo A market is segmented into drugs, enzyme replacement therapy (ERT), gene therapy, genistein, and others. Gene therapy accounted for the largest market share of 38.5% in 2024, attributed to ongoing clinical advancements targeting long-term correction of SGSH mutations.
Genistein is expected to expand at the highest CAGR of 20.3% from 2025 to 2032, due to growing interest in non-invasive and oral alternatives.
• By Mode of Administration
On the basis of mode of administration, the Sanfilippo A market is segmented into injectable, oral, and others. The injectable segment held the largest revenue share of 61.8% in 2024, driven by the dominance of IV-based gene and enzyme therapies.
The oral segment is projected to grow at a CAGR of 17.6% from 2025 to 2032, fueled by pipeline oral drugs and convenience in long-term use.
• By End User
On the basis of end user, the Sanfilippo A market is segmented into hospitals, homecare, specialty clinics, and others. Hospitals captured the largest share of 48.3% in 2024, as they are primary hubs for diagnosis, treatment, and clinical trial access.
Homecare is forecasted to grow at the fastest CAGR of 19.1% between 2025 and 2032, due to patient-centric models and rising use of at-home infusions and genetic monitoring tools.
Sanfilippo A Market Regional Analysis
- North America dominated the Sanfilippo A market with the largest revenue share of 41.6% in 2024, driven by increased awareness of rare diseases, supportive government initiatives, and a high number of ongoing clinical trials. The presence of major biotech companies, well-established healthcare infrastructure, and access to orphan drug designations from regulatory authorities such as the FDA significantly contribute to regional growth
- Consumers in the region increasingly value early diagnosis and access to innovative gene therapy and enzyme replacement solutions. The market is further supported by robust funding from patient advocacy groups and government agencies, making North America a hub for Sanfilippo A research and treatment development
- This widespread development is further backed by high healthcare spending, active rare disease registries, and favorable reimbursement pathways, solidifying the region’s leadership in both diagnosis and experimental therapy access
U.S. Sanfilippo A Market Insight
The U.S. Sanfilippo A market captured the largest revenue share of 83.7% in 2024 within North America, fueled by increased patient screening, clinical research funding, and the rapid advancement of investigational therapies such as AAV-based gene therapy. Growing awareness through advocacy organizations like the Cure Sanfilippo Foundation and rapid expansion of pediatric genetic testing are key growth drivers. Moreover, regulatory incentives such as fast track and breakthrough therapy designations further propel the development pipeline in the U.S.
Europe Sanfilippo A Market Insight
The Europe Sanfilippo A market is projected to expand at a substantial CAGR during the forecast period, driven by pan-European research initiatives, increased genetic testing adoption, and national health system support for rare disease diagnostics and treatment. Enhanced disease surveillance, growing participation in global clinical trials, and increased awareness across healthcare settings are key contributors to regional market expansion.
U.K. Sanfilippo A Market Insight
The U.K. Sanfilippo A market is anticipated to grow at a notable CAGR from 2025 to 2032, supported by initiatives like Genomics England and the NHS’s early access to medicines scheme. Rising public health campaigns around rare pediatric disorders and increased funding for research in neurodegenerative diseases contribute to growth.
Germany Sanfilippo A Market Insight
The Germany Sanfilippo A market is expected to grow at a CAGR during the forecast period, driven by strong pharmaceutical R&D capacity, well-developed healthcare infrastructure, and a supportive regulatory environment for orphan drugs. Patient-centric research networks and partnerships between biotech firms and academic institutions further support clinical progress.
Asia-Pacific Sanfilippo A Market Insight
The Asia-Pacific Sanfilippo A market is projected to grow at the fastest CAGR of 19.4% from 2025 to 2032, due to increasing healthcare investment, rising awareness of rare diseases, and expansion of diagnostic capabilities in countries like China, Japan, and India. Regional initiatives to improve genetic screening and newborn testing are opening new pathways for early detection and clinical care.
Japan Sanfilippo A Market Insight
The Japan Sanfilippo A market is gaining momentum, supported by the country’s emphasis on rare disease registries, advanced clinical infrastructure, and early adoption of gene and enzyme replacement therapy technologies. Japan’s aging caregiver population also underscores the demand for long-term management tools and support systems for families dealing with rare pediatric conditions.
China Sanfilippo A Market Insight
The China Sanfilippo A market accounted for the largest revenue share in Asia-Pacific in 2024, driven by its expanding middle class, government investment in rare disease research, and rising availability of genetic testing. The growth of domestic biotech firms and increased international collaboration on rare disease trials are key factors accelerating market development in China.
Sanfilippo A Market Share
The Sanfilippo A industry is primarily led by well-established companies, including:
- Amgen Inc. (U.S.)
- Lupin (India)
- Sun Pharmaceutical Industries Ltd (India)
- Cipla (India)
- Teva Pharmaceutical Industries Ltd (Israel)
- Alkem (India)
- Hope Pharmaceuticals (U.S.)
- Sanifit (Spain)
- Essity Health & Medical (Germany)
- Viatris Inc. (U.S.)
- Zydus Group (India)
Latest Developments in Global Sanfilippo A Market
- In February 2025, Ultragenyx Pharmaceutical Inc. announced that the U.S. Food and Drug Administration (FDA) has accepted for review its Biologics License Application (BLA) for UX111 (ABO-102), an AAV gene therapy intended to treat patients with Sanfilippo syndrome type A (MPS IIIA), under the accelerated approval pathway. The application was granted Priority Review, with a Prescription Drug User Fee Act (PDUFA) target action date set for August 18, 2025. The FDA also indicated that it does not currently plan to convene an advisory committee meeting to review the application
- In May 2022, Ultragenyx Pharmaceuticals secured licensing rights for an experimental gene therapy aimed at treating Sanfilippo Syndrome. This acquisition strengthens their rare disease pipeline by incorporating innovative treatment options for the condition. The move highlights the company's focus on advancing gene therapy solutions for neurodegenerative disorders
- In January 2022, JCR Pharmaceuticals' drug JR-441 was granted "Orphan Drug" status in Europe for the treatment of Sanfilippo Syndrome. This designation provides the company with additional market exclusivity and regulatory incentives for developing the treatment. The status reinforces JCR's commitment to addressing rare diseases through innovative therapies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.