Global Seminoma Associated Paraneoplastic Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
250.50 Million
USD
387.36 Million
2024
2032
USD
250.50 Million
USD
387.36 Million
2024
2032
| 2025 –2032 | |
| USD 250.50 Million | |
| USD 387.36 Million | |
|
|
|
|
Seminoma-associated Paraneoplastic Syndrome Market Size
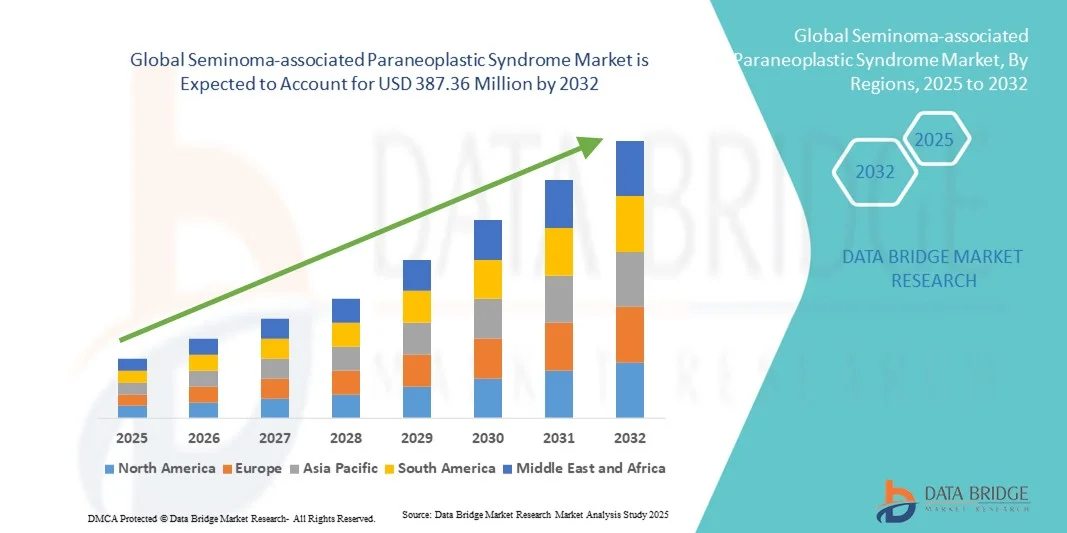
- The global Seminoma-associated Paraneoplastic Syndrome market size was valued at USD 250.5 Million in 2024 and is expected to reach USD 387.36 Million by 2032, at a CAGR of 5.60% during the forecast period
- The market growth is largely fueled by the rising incidence of testicular germ cell tumors, particularly seminoma, and the growing awareness of associated paraneoplastic syndromes that affect multiple organ systems. Advancements in diagnostic imaging, molecular pathology, and biomarker identification are significantly improving early detection and management, driving demand for effective treatment and monitoring solutions across healthcare settings
- Furthermore, the increasing focus on personalized medicine, expanding clinical research into tumor-related autoimmune responses, and the development of novel immunotherapeutic and supportive treatment options are accelerating the uptake of Seminoma-associated Paraneoplastic Syndrome management solutions. These converging factors are substantially boosting the industry’s growth by improving patient outcomes, expanding the diagnostic base, and encouraging collaborations between oncology and neurology specialists worldwide
Seminoma-associated Paraneoplastic Syndrome Market Analysis
- Seminoma-associated Paraneoplastic Syndrome (SPS), a rare complication of testicular germ cell tumors, is gaining growing clinical and research attention due to increasing incidences of seminoma and enhanced understanding of tumor-related autoimmune mechanisms. The market growth is largely driven by advancements in oncological diagnostics, neuroimmunology research, and early recognition of paraneoplastic neurological manifestations, leading to improved patient outcomes and multidisciplinary treatment approaches
- The rising demand for effective immunotherapy, targeted chemotherapy, and supportive neurological care is further fueling market expansion. Ongoing clinical trials investigating immune checkpoint inhibitors, corticosteroid regimens, and combination therapies, along with increased funding for rare cancer research, are supporting the development of novel treatment modalities for seminoma-associated paraneoplastic syndrome
- North America dominated the seminoma-associated paraneoplastic syndrome market with the largest revenue share of 41.8% in 2024, attributed to high healthcare expenditure, early diagnosis rates, and the presence of leading oncology research centers across the U.S. and Canada. The region continues to witness steady growth driven by technological advances in neuro-oncology, access to biologic therapies, and collaborations between cancer institutes and biopharmaceutical firms
- Asia-Pacific is projected to be the fastest-growing region in the seminoma-associated paraneoplastic syndrome market during the forecast period, with an estimated CAGR of 7.6% from 2025 to 2032. This growth is driven by increasing cancer awareness programs, improving healthcare infrastructure, and government investments in rare disease and oncology research, particularly in Japan, China, and South Korea
- The Blood Tests segment held the largest revenue share of 57.4% in 2024, owing to its widespread use in detecting onconeural antibodies such as anti-Hu, anti-Ma2, and anti-Ta
Report Scope and Seminoma-associated Paraneoplastic Syndrome Market Segmentation
|
Attributes |
Seminoma-associated Paraneoplastic Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Seminoma-associated Paraneoplastic Syndrome Market Trends
Enhanced Diagnostic Awareness and Multidisciplinary Integration
- A significant and accelerating trend in the global seminoma-associated paraneoplastic syndrome market is the deepening integration of multidisciplinary diagnostic and therapeutic approaches (oncology, neurology, immunology). This fusion of clinical specialties is significantly enhancing patient identification, intervention timing, and overall disease management
- For instance, emerging biomarker testing such as Kelch‑like Protein 11 Antibody detection in seminoma-associated paraneoplastic neurologic syndromes (PNS) is helping clinicians identify underlying germ-cell tumours earlier and manage the immune-mediated manifestations more effectively
- Integration of advanced imaging, autoantibody panels, coordinated care teams (oncologists + neurologists + immunologists), and patient monitoring tools enables features such as earlier tumour detection, tailored immunotherapy, and more informed prognostic alerting. For example, recognition of anti-Hu or anti-Ma2 antibodies in seminoma patients with neurological symptoms enables targeted work-up and therapy
- Furthermore, the involvement of extended patient registries and multidisciplinary care pathways offers improved coordination, remote monitoring of neurology/oncology status, and holistic intervention planning
- This trend toward more intelligent, integrated, and patient-centric care systems is fundamentally reshaping expectations for management of paraneoplastic syndromes associated with seminoma. Consequently, clinical networks and research consortia are developing pathways combining tumour-eradication, immunotherapy, neurologic rehabilitation and long-term follow-up of PNS in seminoma cohorts
- The demand for clinical pathways that offer seamless coordination between oncology, neurology and immunology—across diagnostics, treatment, follow-up and rehabilitation—is growing rapidly across both hospital and research-centre settings, as healthcare providers increasingly prioritise early detection, functional recovery and comprehensive patient outcomes
Seminoma-associated Paraneoplastic Syndrome Market Dynamics
Driver
Growing Need Due to Rising Recognition of Germ-Cell Tumours and Immune-Mediated Complications
- The increasing recognition of germ-cell tumours (such as seminoma) and their associated paraneoplastic syndromes, coupled with improved awareness of immune-mediated neurological complications, is a significant driver for the Seminoma-associated Paraneoplastic Syndrome market.
- For instance, the identification of Kelch-like protein 11 antibody as a marker in seminoma patients with neurologic manifestations has spurred new diagnostic and treatment pathway development
- As clinicians become more aware of the importance of early recognition of paraneoplastic neurologic syndromes (PNS) in seminoma patients, the adoption of integrated diagnostics (autoantibody assays, advanced imaging, tumour screening) has increased substantially.
- Furthermore, the growing interest in precision medicine, immunotherapy, and neurologic rehabilitation is fostering the development of new treatment models targeting both the tumour and the immune-mediated syndrome
- The convenience and potential for improved functional outcomes—via timely diagnosis and integrated care—are key factors propelling adoption of these advanced care pathways in both oncology and neurology settings. The trend towards patient-centred care and availability of multidisciplinary treatment programmes further contribute to market growth
Restraint/Challenge
Limited Awareness, Diagnostic Complexity and High Care-Coordination Costs
- The rarity of seminoma-associated paraneoplastic syndromes and limited awareness among many clinicians remain key barriers to broad market growth. Because the neurologic symptoms often precede tumour diagnosis or mimic other neurologic disorders, diagnosis is frequently delayed or mis-assigned
- For instance, paraneoplastic neurologic syndromes may present months before the underlying tumour is found, complicating timely intervention and increasing the risk of irreversible neurologic damage
- The complexity of care coordination—requiring oncologists, neurologists, immunologists, diagnostic labs, and rehabilitation services—adds to cost, logistical burden and may limit access, especially in resource-constrained settings
- In addition, because standardised therapeutic protocols for seminoma-associated PNS are still evolving, with limited large-scale trials and approved therapies in many jurisdictions, patient access and confidence may be hindered
- Overcoming these challenges will require expanded clinician education, streamlined diagnostic pathways, enhanced multidisciplinary networks, increased reimbursement support and broader access to specialised care
Seminoma-associated Paraneoplastic Syndrome Market Scope
The market is segmented on the basis of treatment, symptoms, diagnosis, end-users, and distribution channel.
- By Treatment
On the basis of treatment, the Seminoma-associated Paraneoplastic Syndrome market is segmented into Corticosteroids, Immunosuppressant, Intravenous Immunoglobulin (IVIg), Chemotherapy, Surgery, Physical Therapy, Speech Therapy, and Others. The Corticosteroids segment dominated the market with the largest revenue share of 39.5% in 2024, owing to their proven efficacy in reducing inflammation and immune-mediated neuronal damage associated with paraneoplastic neurological syndromes. Corticosteroids remain the first-line therapy for symptom management, helping to suppress autoimmune responses triggered by tumor antigens. The segment continues to gain traction due to wide clinical acceptance, cost-effectiveness, and accessibility across healthcare systems globally. Furthermore, the ability of corticosteroids to stabilize neurological function in early-stage cases ensures consistent demand, especially in hospital and specialty care settings.
The Immunosuppressant segment is anticipated to witness the fastest growth rate of 18.9% from 2025 to 2032, driven by the rising preference for advanced immunotherapy approaches such as azathioprine, mycophenolate mofetil, and cyclosporine. These agents offer durable disease control and improved neurological outcomes when corticosteroids prove insufficient or are contraindicated. Increasing adoption of combined immunosuppressive regimens and ongoing research into targeted immunomodulatory drugs are accelerating market expansion. The emergence of personalized medicine and integration of immunosuppressants in multidisciplinary treatment protocols are expected to fuel significant long-term growth.
- By Symptoms
On the basis of symptoms, the Seminoma-associated Paraneoplastic Syndrome market is segmented into Reduced Reflexes, Speech Difficulty, Progressive Amnesia, Psychiatric Disturbances, Double Vision, Headache, and Others. The Reduced Reflexes segment dominated the largest market revenue share of 32.6% in 2024, attributed to its high prevalence among affected patients and its diagnostic value in early clinical evaluation. Reduced reflexes serve as a key neurological marker for peripheral nerve involvement, prompting further imaging and serological testing. The strong emphasis on early symptom detection in neurology departments and increasing awareness among clinicians about paraneoplastic syndromes have strengthened the segment’s position. Rising global caseloads of germ cell tumors associated with neurological manifestations further sustain its dominance.
The Speech Difficulty segment is projected to witness the fastest CAGR of 19.1% from 2025 to 2032, driven by the growing incidence of cerebellar and limbic system involvement in paraneoplastic encephalitis. Speech impairments often necessitate specialized rehabilitative therapies, including speech and language intervention, spurring demand in clinical and rehabilitation centers. The expansion of multidisciplinary care models integrating neurologists, oncologists, and speech therapists is fueling treatment adoption. Increasing awareness and improved diagnostic accuracy for speech-related neurological dysfunctions are expected to contribute to strong market growth through 2032.
- By Diagnosis
On the basis of diagnosis, the Seminoma-associated Paraneoplastic Syndrome market is segmented into Blood Tests and Imaging Tests. The Blood Tests segment held the largest revenue share of 57.4% in 2024, owing to its widespread use in detecting onconeural antibodies such as anti-Hu, anti-Ma2, and anti-Ta. Blood-based diagnostics offer rapid, minimally invasive screening, making them the preferred initial diagnostic approach for patients presenting with neurological symptoms linked to seminomas. The increasing adoption of advanced serological assays, including ELISA and immunoblot techniques, enhances detection accuracy and patient monitoring efficiency. Growing clinical collaborations for biomarker validation continue to drive the segment’s leading position.
The Imaging Tests segment is expected to witness the fastest growth rate of 20.4% from 2025 to 2032, supported by advancements in MRI and PET-CT technologies that allow precise localization of tumors and neural lesions. Non-invasive imaging offers valuable insights into disease progression and therapeutic response. AI-powered imaging analytics are improving diagnostic precision and reducing interpretation time. Growing hospital investments in high-resolution diagnostic equipment and increasing reliance on multimodal imaging for neurological evaluation are expected to fuel robust expansion throughout the forecast period.
- By End-Users
On the basis of end-users, the Seminoma-associated Paraneoplastic Syndrome market is segmented into Clinics, Hospitals, Diagnostic Centres, Surgical Centres, and Others. The Hospitals segment accounted for the largest revenue share of 48.7% in 2024, driven by the availability of comprehensive diagnostic and therapeutic infrastructure. Hospitals are the primary sites for chemotherapy, surgery, and corticosteroid administration, enabling efficient management of complex paraneoplastic cases. The presence of multidisciplinary teams combining oncology, neurology, and immunology expertise enhances patient outcomes and facilitates advanced care. Increasing hospital admissions for germ cell tumor-associated neurological syndromes further strengthen this segment’s dominance.
The Diagnostic Centres segment is projected to record the fastest CAGR of 17.8% from 2025 to 2032, owing to growing demand for specialized laboratory testing and imaging services. Technological advancements in antibody testing and MRI interpretation are enabling early detection and differentiation of paraneoplastic manifestations. Rising collaborations between diagnostic laboratories and oncology clinics are expanding accessibility to advanced tests. The growing preference for outpatient diagnosis and the emergence of dedicated neuro-immunology labs are anticipated to drive segmental growth.
- By Distribution Channel
On the basis of distribution channel, the Seminoma-associated Paraneoplastic Syndrome market is segmented into Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy. The Hospital Pharmacy segment dominated the market with the largest revenue share of 52.9% in 2024, supported by the hospital-centric nature of treatment administration. Complex therapies such as corticosteroids, immunosuppressants, and intravenous immunoglobulins are primarily dispensed through hospital pharmacies, ensuring proper monitoring and dosage control. The centralization of procurement processes and integrated supply chains across tertiary hospitals further reinforce this dominance.
The Online Pharmacy segment is projected to witness the fastest growth rate of 21.6% from 2025 to 2032, fueled by rising digitalization in healthcare and increasing patient inclination toward home delivery of supportive medications. The growing presence of e-pharmacy platforms offering neurological and immunotherapy drugs, along with favorable regulatory reforms in telehealth, is supporting rapid expansion. The segment benefits from enhanced convenience, broader drug availability, and growing awareness of online therapeutic access for rare disorders. In addition, increasing collaborations between pharmaceutical manufacturers and online distributors are expected to streamline medicine accessibility and ensure timely delivery to patients worldwide.
Seminoma-associated Paraneoplastic Syndrome Market Regional Analysis
- North America dominated the seminoma-associated paraneoplastic syndrome market with the largest revenue share of 41.8% in 2024, attributed to high healthcare expenditure, early diagnosis rates, and the presence of leading oncology research centers across the U.S. and Canada. The region continues to witness steady growth driven by technological advances in neuro-oncology, access to biologic and immunomodulatory therapies, and collaborations between cancer institutes and biopharmaceutical companies
- Continuous expansion of rare disease registries and clinical trial networks, particularly within the U.S., supports faster diagnosis and treatment development for paraneoplastic disorders. Increasing government funding and patient advocacy initiatives are further accelerating awareness and early clinical intervention
- The market accounts for the majority share of the North American market due to its robust healthcare infrastructure, widespread access to tertiary care hospitals, and the availability of advanced diagnostic tools such as antibody profiling and imaging systems for tumour detection
U.S. Seminoma-associated Paraneoplastic Syndrome Market Insight
The U.S. seminoma-associated paraneoplastic syndrome market captured of the regional share in 2024, primarily driven by an advanced healthcare ecosystem and strong emphasis on early detection of cancer-related neurological disorders. The country benefits from active collaborations among cancer research institutions, academic hospitals, and biotech firms working on immunotherapy and neuro-oncology integration. Furthermore, the implementation of clinical practice guidelines for paraneoplastic syndromes by leading bodies such as the National Comprehensive Cancer Network (NCCN) and the American Academy of Neurology (AAN) has strengthened standard-of-care approaches. Increasing adoption of antibody-based diagnostic panels, precision oncology initiatives, and patient registry participation further propel the U.S. market growth trajectory.
Europe Seminoma-associated Paraneoplastic Syndrome Market Insight
The Europe seminoma-associated paraneoplastic syndrome market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising cancer prevalence, growing awareness of rare oncologic syndromes, and the adoption of personalized medicine approaches. The region’s healthcare systems emphasize multidisciplinary management, integrating oncologists, neurologists, and immunologists to optimize outcomes. Supportive regulatory frameworks for orphan diseases, along with significant investments by the European Medicines Agency (EMA) in rare disease programs, are expected to foster steady market expansion. Countries such as Germany, France, and the U.K. are leading contributors to regional growth, supported by high-quality diagnostic laboratories and access to targeted therapies.
U.K. Seminoma-associated Paraneoplastic Syndrome Market Insight
The U.K. seminoma-associated paraneoplastic syndrome market is anticipated to grow at a noteworthy CAGR during 2025–2032, driven by government-led rare disease initiatives, the expansion of genomic medicine, and the availability of specialized neurology and oncology centers. Increasing adoption of early antibody screening and advanced imaging technologies is improving detection accuracy and treatment planning. The U.K.’s strong emphasis on translational research, coupled with National Health Service (NHS) funding for complex cancer care, continues to enhance diagnosis and patient outcomes.
Germany Seminoma-associated Paraneoplastic Syndrome Market Insight
The Germany seminoma-associated paraneoplastic syndrome market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing clinical recognition of paraneoplastic manifestations in germ-cell tumours and technological advances in diagnostic tools. Germany’s robust healthcare infrastructure, significant investment in oncology research, and strong academic–industry collaboration contribute to diagnostic and therapeutic innovation. Furthermore, high patient awareness and accessibility to comprehensive cancer care centres are supporting continuous market expansion.
Asia-Pacific Seminoma-associated Paraneoplastic Syndrome Market Insight
The Asia-Pacific seminoma-associated paraneoplastic syndrome market is poised to grow at the fastest CAGR of 7.6% from 2025 to 2032, driven by increasing cancer awareness, improving healthcare infrastructure, and growing investments in rare disease and oncology research—particularly in Japan, China, and South Korea. Government support through national cancer control programs, the establishment of rare disease registries, and the rising availability of antibody testing and imaging facilities are propelling early diagnosis and treatment adoption. In addition, international collaborations and clinical trial expansion across the region are strengthening research pipelines.
Japan Seminoma-associated Paraneoplastic Syndrome Market Insight
The Japan Seminoma-associated Paraneoplastic Syndrome market is gaining momentum due to high diagnostic precision, advanced imaging technology, and government funding in rare cancer programs. The presence of leading academic hospitals and a well-coordinated national rare disease policy framework drive research participation and early clinical identification. In addition, growing patient awareness and integration of molecular diagnostics in oncology and neurology departments further contribute to Japan’s strong growth outlook during the forecast period.
China Seminoma-associated Paraneoplastic Syndrome Market Insight
The China seminoma-associated paraneoplastic syndrome market accounted for the largest share within Asia-Pacific in 2024, driven by government-backed rare disease programs, rapid expansion of tertiary hospitals, and technological upgrades in endoscopic and laboratory facilities. Ongoing national healthcare reforms, investment in diagnostic infrastructure, and increased participation in international oncology research networks are bolstering market expansion. Furthermore, domestic biopharma firms’ entry into paraneoplastic immunotherapy development enhances access and affordability for patients across major urban centres.
Seminoma-associated Paraneoplastic Syndrome Market Share
The Seminoma-associated Paraneoplastic Syndrome industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- Sanofi S.A. (France)
- Johnson & Johnson (U.S.)
- AstraZeneca plc (U.K.)
- Bayer AG (Germany)
- GlaxoSmithKline plc (U.K.)
- Merck & Co., Inc. (U.S.)
- Bristol Myers Squibb (U.S.)
- AbbVie Inc. (U.S.)
- Amgen Inc. (U.S.)
- Roche Holding AG (Switzerland)
- Takeda Pharmaceutical Company Limited (Japan)
- Lilly (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
Latest Developments in Global Seminoma-associated Paraneoplastic Syndrome Market
- In July 2021, updated international diagnostic criteria for Paraneoplastic Neurologic Syndromes (PNS) were officially released by an expert panel of neurologists and oncologists. These new guidelines standardized antibody classifications, tumor-association scoring systems, and diagnostic procedures, improving early identification and management of seminoma-associated PNS worldwide. This update has been instrumental in advancing research and clinical recognition of paraneoplastic manifestations in germ cell tumors
- In July 2022, researchers reported a rare case of Paraneoplastic Tumefactive Demyelination Associated with Seminoma in a 32-year-old Japanese male. The case broadened understanding of seminoma-induced neurological complications and demonstrated how immune-mediated demyelination can mimic multiple sclerosis, emphasizing the importance of immunological testing and timely intervention. This discovery prompted renewed clinical attention to early neurological screening in seminoma patients
- In September 2023, a detailed clinical report described a case of Primary Mediastinal Seminoma Presenting with Paraneoplastic Anti-Hu Encephalitis in a 19-year-old male. This highlighted the expanding spectrum of autoimmune neurological syndromes linked to seminoma, particularly those mediated by anti-neuronal antibodies. The findings reinforced the need for antibody screening in patients with germ cell tumors and unexplained neurological symptoms
- In April 2024, a comprehensive review titled “Testicular Cancer and Paraneoplastic Encephalitis” was published, summarizing multiple clinical cases and recent advances in diagnosis and treatment. The study identified seminoma as one of the primary germ cell tumors associated with autoimmune encephalitis, driving awareness among oncologists and neurologists. The publication also discussed the increasing use of immunotherapy and corticosteroids in managing seminoma-related neurological complications
- In May 2025, an immunology research study titled “Mechanisms of Autoimmune-Mediated Paraneoplastic Syndromes: Immune Tolerance and Disease Pathogenesis” was released, shedding new light on how ectopic antigen expression in seminoma tissue can trigger immune cross-reactivity against the nervous system. The study contributed significantly to the understanding of disease mechanisms and opened avenues for the development of targeted immunomodulatory treatments
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.