Global Sennetsu Fever Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
479.48 Million
USD
651.18 Million
2025
2033
USD
479.48 Million
USD
651.18 Million
2025
2033
| 2026 –2033 | |
| USD 479.48 Million | |
| USD 651.18 Million | |
|
|
|
|
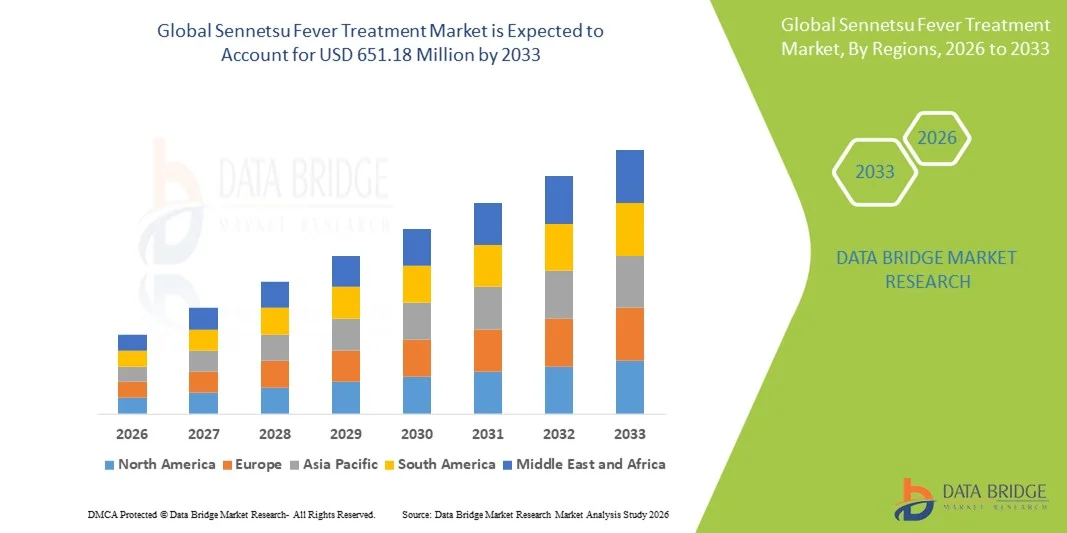
Sennetsu Fever Treatment Market Size
- The global Sennetsu fever treatment market size was valued at USD 479.48 million in 2025 and is expected to reach USD 651.18 million by 2033, at a CAGR of 3.90% during the forecast period
- The market growth is largely fueled by rising awareness of Sennetsu fever in endemic regions and the increasing emphasis on early diagnosis and effective therapeutic management, supported by improved surveillance systems and healthcare infrastructure advancements
- Furthermore, growing demand for reliable, accessible, and targeted treatment options including antimicrobial therapies and supportive care is positioning Sennetsu fever treatment as a critical focus area within infectious disease management. These converging factors are accelerating the adoption of Sennetsu fever treatment solutions, thereby significantly boosting the industry's growth
Sennetsu Fever Treatment Market Analysis
- Sennetsu fever treatment, focused on managing an infection caused by Neorickettsia sennetsu, is becoming increasingly important within infectious disease care due to the need for accurate diagnosis, timely antimicrobial therapy, and improved clinical awareness in both endemic and emerging risk regions, supporting better patient outcomes and reduced complications
- The escalating demand for effective Sennetsu fever treatment is primarily fueled by rising detection rates enabled by improved diagnostic technologies, expanding infectious disease surveillance systems, and increasing clinical recognition of the condition, leading to stronger emphasis on timely and targeted therapeutic interventions
- North America dominated the Sennetsu fever treatment market with the largest revenue share of 41.8% in 2025, characterized by advanced healthcare infrastructure, strong infectious disease management frameworks, and heightened diagnostic capabilities, with the U.S. showing substantial growth in treatment adoption driven by increased clinician awareness and improvements in laboratory testing accuracy
- Asia-Pacific is expected to be the fastest growing region in the Sennetsu fever treatment market during the forecast period due to rising healthcare investments, expanding access to infectious disease specialists, and growing awareness initiatives aimed at improving diagnosis and treatment in countries with emerging or under-recognized disease prevalence
- The antibiotics segment dominated the Sennetsu fever treatment market with a market share of 58.9% in 2025, driven by the proven effectiveness of tetracycline-based regimens, widespread availability of antimicrobial drugs, and strong physician preference for standardized clinical protocols that ensure consistent and reliable patient recovery outcomes
Report Scope and Sennetsu Fever Treatment Market Segmentation
|
Attributes |
Sennetsu Fever Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Sennetsu Fever Treatment Market Trends
Advancement in Molecular Diagnostics and AI-Assisted Disease Detection
- A significant and accelerating trend in the global Sennetsu fever treatment market is the rapid advancement in molecular diagnostics and the emerging integration of AI-supported infectious disease detection systems, which are greatly improving accuracy, enabling earlier intervention, and enhancing clinical decision-making for healthcare providers
- For instance, several PCR-based diagnostic platforms are being upgraded with automated detection software capable of identifying Neorickettsia sennetsu with higher sensitivity, allowing clinicians to confirm cases more efficiently and initiate timely treatment
- AI integration in infectious disease diagnostics enables algorithms to analyze clinical data patterns, flag atypical presentations, and support faster differential diagnosis. For instance, some hospital systems in Japan are piloting AI-assisted laboratory workflows to detect rickettsial infections more precisely and reduce diagnostic turnaround time, thereby supporting improved patient management
- The seamless integration of advanced diagnostics with electronic health records is enabling unified patient monitoring, allowing clinicians to track symptoms, lab results, and treatment outcomes in one platform, creating a more cohesive infectious disease management ecosystem
- This trend toward more intelligent, precise, and interconnected diagnostic solutions is fundamentally reshaping expectations for early detection and treatment of rickettsial diseases. Consequently, companies specializing in infectious disease diagnostics are developing enhanced molecular panels with automated interpretation to support clinicians treating Sennetsu fever
- The demand for accurate, rapid, and AI-supported diagnostic solutions is growing rapidly across hospitals and research institutions, as healthcare systems increasingly prioritize early detection and comprehensive infectious disease management
Sennetsu Fever Treatment Market Dynamics
Driver
Growing Need Due to Rising Disease Detection and Diagnostic Improvements
- The increasing recognition of Sennetsu fever due to expanding diagnostic capabilities, coupled with the growing emphasis on infectious disease surveillance, is a significant driver supporting rising demand for effective treatment solutions worldwide
- For instance, in April 2025, several regional health agencies in East Asia expanded their molecular testing programs for rickettsial infections, integrating high-sensitivity PCR tools into surveillance frameworks. Such initiatives are expected to drive the Sennetsu fever treatment market growth throughout the forecast period
- As clinicians become more aware of the disease’s clinical presentation and associated risks, timely diagnosis and treatment are becoming a priority, with antimicrobial therapies offering a major improvement over untreated disease progression
- Furthermore, the increasing focus on strengthening public health systems and laboratory capacities in both developed and emerging regions is making Sennetsu fever treatment a more prominent component of infectious disease preparedness strategies
- The availability of standardized treatment guidelines, early therapeutic interventions, and improved antibiotic accessibility are key factors propelling treatment adoption across hospitals and clinics. The trend toward integrating diagnosis-to-treatment pathways within infectious disease programs further contributes to market growth
Restraint/Challenge
Limited Clinical Awareness and Diagnostic Accessibility Hurdles
- Concerns surrounding limited clinical awareness of Sennetsu fever among healthcare providers, especially in non-endemic or low-surveillance regions, pose a significant challenge to broader treatment adoption, as underdiagnosis results in missed therapeutic opportunities
- For instance, reports of misdiagnosed rickettsial infections in certain regions have highlighted gaps in clinician training and laboratory capacity, causing delays in identifying Sennetsu fever cases and initiating appropriate antimicrobial therapy
- Addressing these challenges through improved clinical education programs, wider deployment of molecular tests, and stronger infectious disease training is crucial for improving case detection. Health institutes in affected regions emphasize diagnostic modernization and medical workforce training to enhance accuracy and response
- In addition, the relatively limited availability of advanced diagnostic tools in rural or resource-constrained areas can delay confirmation of Sennetsu fever, restricting timely access to standardized treatment protocols. While basic antibiotics remain accessible, advanced diagnostics still require infrastructural investment
- While diagnostic capabilities are gradually improving, the uneven distribution of laboratory resources continues to hinder widespread detection, especially in underserved regions
- Overcoming these challenges through expanded surveillance, better clinician training, and improved availability of rapid diagnostic solutions will be vital for sustained market growth
Sennetsu Fever Treatment Market Scope
The market is segmented on the basis of treatment, diagnosis, dosage, route of administration, end-users, and distribution channel.
- By Treatment
On the basis of treatment, the Sennetsu fever treatment market is segmented into antibiotics and others. The antibiotics segment dominated the market with the largest market revenue share of 58.9% in 2025, driven by their established clinical effectiveness in treating infections caused by Neorickettsia sennetsu. Antibiotics, particularly doxycycline, are widely recognized as first-line therapy and are consistently recommended in infectious disease guidelines. Hospitals and clinics rely heavily on antibiotic therapy due to its proven ability to rapidly control symptoms and prevent complications. Growing diagnostic accuracy is further increasing the timely use of antibiotics, strengthening their dominance. The availability of generic formulations also boosts accessibility across developing and developed regions.
The “others” segment is anticipated to witness the fastest growth rate from 2026 to 2033, fueled by increasing emphasis on supportive care to manage systemic symptoms associated with Sennetsu fever. Supportive therapies such as antipyretics and hydration management are being used more frequently to improve patient comfort and recovery time. For instance, clinicians in endemic regions are adopting complementary therapies alongside standard antibiotics for better outcomes. The growing focus on comprehensive febrile illness management is also contributing to the rising adoption of these treatments. Increasing awareness about symptom-based care further accelerates segment growth.
- By Diagnosis
On the basis of diagnosis, the market is segmented into blood tests, liver function tests, indirect immunofluorescent assay (IFA), PCR test, and others. The PCR test segment held the largest market revenue share in 2025, driven by its superior accuracy and ability to detect Neorickettsia sennetsu in the early stages of infection. PCR testing is widely used in hospitals equipped with advanced molecular diagnostic systems, enabling timely confirmation and rapid initiation of treatment. For instance, healthcare facilities in East Asia increasingly rely on PCR panels for rickettsial detection due to their sensitivity and quick turnaround. The integration of PCR into infectious disease surveillance programs further strengthens its dominance. Growing investments in laboratory infrastructure also support increased adoption.
The IFA segment is expected to witness the fastest CAGR from 2026 to 2033, driven by its growing use for serological confirmation in cases where PCR testing is unavailable or delayed. IFA is becoming increasingly valuable in resource-limited settings due to its affordability and reliability for antibody detection. For instance, public health laboratories in Asia-Pacific are expanding IFA capabilities as part of rickettsial disease monitoring programs. Its role in epidemiological analysis and retrospective diagnosis makes it an important complement to molecular testing. These factors contribute to its accelerating growth.
- By Dosage
On the basis of dosage, the market is segmented into injection, tablet, and others. The tablet segment dominated the market with the largest revenue share in 2025, driven by the widespread use of oral doxycycline and related antibiotics as first-line treatment for Sennetsu fever. Oral medications are easy to administer, cost-effective, and suitable for outpatient care, making them highly preferred in both hospitals and clinics. Tablets are readily available across retail and hospital pharmacies, supporting broader treatment access. For instance, national treatment guidelines in endemic regions commonly prioritize oral antibiotic courses for uncomplicated cases. Growing awareness and early diagnosis further reinforce the dominance of tablet dosage forms.
The injection segment is expected to witness the fastest growth rate from 2026 to 2033, fueled by increasing use in severe or complicated Sennetsu fever cases requiring rapid therapeutic action. Injectable formulations allow immediate drug availability, making them essential for patients unable to tolerate oral medications. For instance, tertiary hospitals increasingly use IV antibiotics for patients presenting with acute symptoms or complications. Expanding critical care capacity in developing regions also contributes to rising adoption. The growing focus on emergency infectious disease management strengthens the segment’s growth trajectory.
- By Route of Administration
On the basis of route of administration, the market is segmented into intravenous, oral, and others. The oral segment dominated the market with the largest revenue share in 2025, driven by the convenience, safety, and adherence advantages of orally administered antibiotics. Oral therapy is the preferred route for most Sennetsu fever patients and is widely prescribed in outpatient and primary care settings. For instance, healthcare providers in endemic regions commonly initiate treatment with oral doxycycline following diagnosis. The affordability and accessibility of oral medications further increase patient uptake. Growing emphasis on early intervention also ensures strong demand for oral routes.
The intravenous segment is anticipated to witness the fastest growth rate from 2026 to 2033, fueled by its increasing use in severe or hospitalized cases requiring rapid therapeutic response. IV administration ensures consistent absorption and immediate effect, making it critical in advanced infections. For instance, hospitals with dedicated infectious disease units are expanding the use of IV antibiotics for acute febrile conditions. Improvements in inpatient care facilities and rising awareness of severe complications are also contributing to IV route adoption. The segment is expected to expand significantly with increased emergency care preparedness.
- By End-Users
On the basis of end-users, the market is segmented into clinic, hospital, and others. The hospital segment dominated the market with the largest revenue share in 2025, driven by the need for advanced diagnostics, specialist evaluation, and treatment monitoring. Hospitals are equipped with PCR testing capabilities, enabling accurate detection and immediate response to confirmed cases. For instance, infectious disease departments in major hospitals manage the majority of rickettsial fever cases requiring laboratory assessment. Hospitals also handle complications such as liver involvement, strengthening their central role in patient care. Their participation in public health surveillance programs further reinforces dominance.
The clinic segment is expected to witness the fastest growth rate from 2026 to 2033, fueled by rising patient preference for accessible and affordable outpatient care. Clinics are increasingly adopting streamlined diagnostic pathways for febrile illnesses and providing quicker consultations. For instance, primary care clinics in Asia-Pacific are integrating rapid tests and treatment protocols for rickettsial infections. Growing decentralization of healthcare delivery and increased awareness of early symptoms contribute to clinic segment expansion. The shift toward community-based disease management supports sustained growth.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market with the largest revenue share in 2025, driven by their central role in dispensing antibiotics required immediately after diagnosis. Hospital pharmacies maintain essential stock levels of recommended antimicrobial therapies and supportive medications. For instance, treatment units in hospitals routinely coordinate with hospital pharmacies to ensure timely administration of prescribed antibiotics. Their integration into hospital workflows and infectious disease programs strengthens reliability and dominance.
The online pharmacy segment is anticipated to witness the fastest growth rate from 2026 to 2033, fueled by rising digital health adoption and growing acceptance of e-prescription services. Online pharmacies offer convenient access to medications, especially for patients receiving outpatient treatment. For instance, national e-pharmacy platforms are increasingly expanding coverage of prescription antibiotics and supportive care drugs. The popularity of home delivery services and increased digital literacy further support market expansion. Online channels are expected to grow rapidly as telehealth adoption continues.
Sennetsu Fever Treatment Market Regional Analysis
- North America dominated the Sennetsu fever treatment market with the largest revenue share of 41.8% in 2025, characterized by advanced healthcare infrastructure, strong infectious disease management frameworks, and heightened diagnostic capabilities, with the U.S. showing substantial growth in treatment adoption driven by increased clinician awareness and improvements in laboratory testing accuracy
- The region’s dominance is driven by widespread adoption of molecular diagnostic tools such as PCR and IFA, enabling faster and more accurate detection of rare rickettsial infections such as Sennetsu fever
- In addition, high healthcare spending, well-established hospital networks, and strong awareness among clinicians regarding atypical febrile illnesses support early treatment and greater uptake of antibiotic therapies, reinforcing North America’s leadership in the market
U.S. Sennetsu Fever Treatment Market Insight
The U.S. Sennetsu fever treatment market captured the largest revenue share within North America in 2025, supported by advanced infectious disease monitoring systems and high adoption of molecular diagnostic tools such as PCR and IFA. Clinician awareness of rare rickettsial infections remains comparatively high due to strong medical training and established reporting channels. The integration of cutting-edge laboratory infrastructure, strong pharmaceutical availability, and rapid access to antibiotic therapy further drive the market. In addition, ongoing research collaborations and improved travel-related disease tracking contribute to early detection, timely treatment, and continued market expansion.
Europe Sennetsu Fever Treatment Market Insight
The Europe Sennetsu fever treatment market is projected to grow steadily throughout the forecast period, driven by the region’s stringent infectious disease surveillance policies and strong public health systems. Increasing focus on identifying rare and emerging pathogens is boosting the use of advanced diagnostics across laboratories. Rising collaboration among European research institutes is also contributing to improved understanding and recognition of rickettsial infections. Moreover, growing investment in healthcare infrastructure, supportive reimbursement systems, and high patient access to antibiotic therapies are fostering sustained market growth.
U.K. Sennetsu Fever Treatment Market Insight
The U.K. Sennetsu fever treatment market is expected to grow at a notable CAGR, propelled by the nation’s strong emphasis on infectious disease management and advanced diagnostic capabilities. Expanded use of PCR-based testing and high clinician vigilance toward atypical febrile illnesses supports early detection and prompt treatment. Travel-associated cases and increased diagnostic sensitivity are further raising awareness of rickettsial infections. Continued government investment in laboratory networks and streamlined access to antibiotic treatment options also support the market’s upward trajectory.
Germany Sennetsu Fever Treatment Market Insight
The Germany Sennetsu fever treatment market is anticipated to witness considerable expansion, supported by high adoption of precision diagnostics and the country’s long-standing focus on healthcare innovation. German laboratories are equipped with advanced molecular technologies, enabling faster identification of rare pathogens. In addition, strong public health programs and a well-structured hospital system ensure timely treatment using evidence-based antibiotic therapies. With rising attention to imported infectious diseases and enhanced surveillance systems, Germany continues to strengthen its diagnostic and treatment ecosystem.
Asia-Pacific Sennetsu Fever Treatment Market Insight
The Asia-Pacific Sennetsu fever treatment market is projected to grow at the fastest CAGR during the forecast period, driven by the presence of endemic regions, growing healthcare awareness, and expanding diagnostic capacity. Countries such as Japan, Malaysia, and Thailand are investing heavily in infectious disease control and laboratory development. Increasing access to PCR and serological testing is enhancing case identification, while rising healthcare expenditure and broadening pharmaceutical distribution networks support treatment uptake. Government-led disease prevention programs and the digitalization of healthcare services are further accelerating regional growth.
Japan Sennetsu Fever Treatment Market Insight
The Japan Sennetsu fever treatment market holds significant importance as the country represents one of the primary endemic areas historically associated with the disease. High public health awareness, advanced medical infrastructure, and extensive use of molecular diagnostic tools contribute to strong disease monitoring and early treatment initiation. Japan’s emphasis on research and epidemiological tracking supports continuous improvements in diagnostic accuracy. In addition, growing healthcare digitalization and declining barriers to antibiotic access are strengthening treatment adoption across both urban and rural settings.
India Sennetsu Fever Treatment Market Insight
The India Sennetsu fever treatment market is expanding steadily, driven by increasing diagnostic modernization, improvements in infectious disease awareness, and the rapid strengthening of laboratory infrastructure. Although Sennetsu fever cases remain rare, rising surveillance of febrile illnesses and broader access to PCR-based testing are improving detection capability. India’s large healthcare ecosystem, expanding pharmaceutical manufacturing strength, and growing focus on rural health programs contribute to improved treatment availability. Increasing clinician training and government-backed public health initiatives continue to support market growth.
Sennetsu Fever Treatment Market Share
The Sennetsu Fever Treatment industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- GSK plc (U.K.)
- AstraZeneca (U.K.)
- Sanofi (France)
- Bayer AG (Germany)
- Johnson & Johnson Services, Inc. (U.S.)
- Novartis AG (Switzerland)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Abbott (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Cipla Limited (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Dr. Reddy's Laboratories Ltd. (India)
- BIOMÉRIEUX (France)
- Thermo Fisher Scientific Inc. (U.S.)
- QIAGEN. (Netherlands)
- BD (U.S.)
What are the Recent Developments in Global Sennetsu Fever Treatment Market?
- In January 2025, a bioinformatics study proposed a potential mechanistic overlap between immune signatures observed in Sennetsu fever and certain COVID-19-related hepatic and hematological abnormalities, based on computational modeling. This raises new scientific questions about Sennetsu fever pathology, enabling future therapeutic investigations into shared inflammatory markers or treatment targets
- In July 2024, a large-scale zoonotic pathogen surveillance project in bats identified multiple Neorickettsia-positive samples, emphasizing rising global attention on emerging Anaplasmataceae infections. The study reinforced a One-Health approach, highlighting how environmental, animal, and human health interactions may influence the emergence of Sennetsu-such as diseases
- In February 2024, researchers reported high genetic diversity of Neorickettsia species including strains genetically related to N. sennetsu in vampire bats in Brazil, indicating that the pathogen’s ecological range is wider than previously believed. The study identified Neorickettsia DNA in bat tissues using next-generation sequencing techniques
- In September 2023, molecular biologists detected Neorickettsia spp. in wild coatis (Nasua nasua) in Brazil, using sequencing and phylogenetic analysis to confirm presence of these bacteria in non-traditional hosts. This finding broadens the suspected transmission ecology of Neorickettsia infections including Sennetsu fever by showing that additional wildlife species may act as reservoirs
- In March 2023, researchers developed and validated an enhanced real-time PCR assay targeting the Ssa2 gene of Neorickettsia, providing improved sensitivity for detecting infections, especially at low bacterial loads. Although primarily validated for N. risticii, the close genetic similarity within the genus suggests that this assay framework could be adapted for N. sennetsu diagnostics
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.