Global Sepsis Market
Market Size in USD Billion
CAGR :
% 
 USD
1.07 Billion
USD
1.98 Billion
2023
2031
USD
1.07 Billion
USD
1.98 Billion
2023
2031
| 2024 –2031 | |
| USD 1.07 Billion | |
| USD 1.98 Billion | |
|
|
|
|
Market Analysis and Size
Surging cases of various infectious diseases which results in growing sepsis prevalence is estimated to drive the market’s growth across the globe. The sepsis market is largely influenced by surging focus of key players towards technological advances in the field of molecular diagnostics and indulging towards collaboration and partnerships with other organizations. Owing to the presence of various growth determinants the market is being propelled forward and is projected to show substantial growth over the forecasted period.
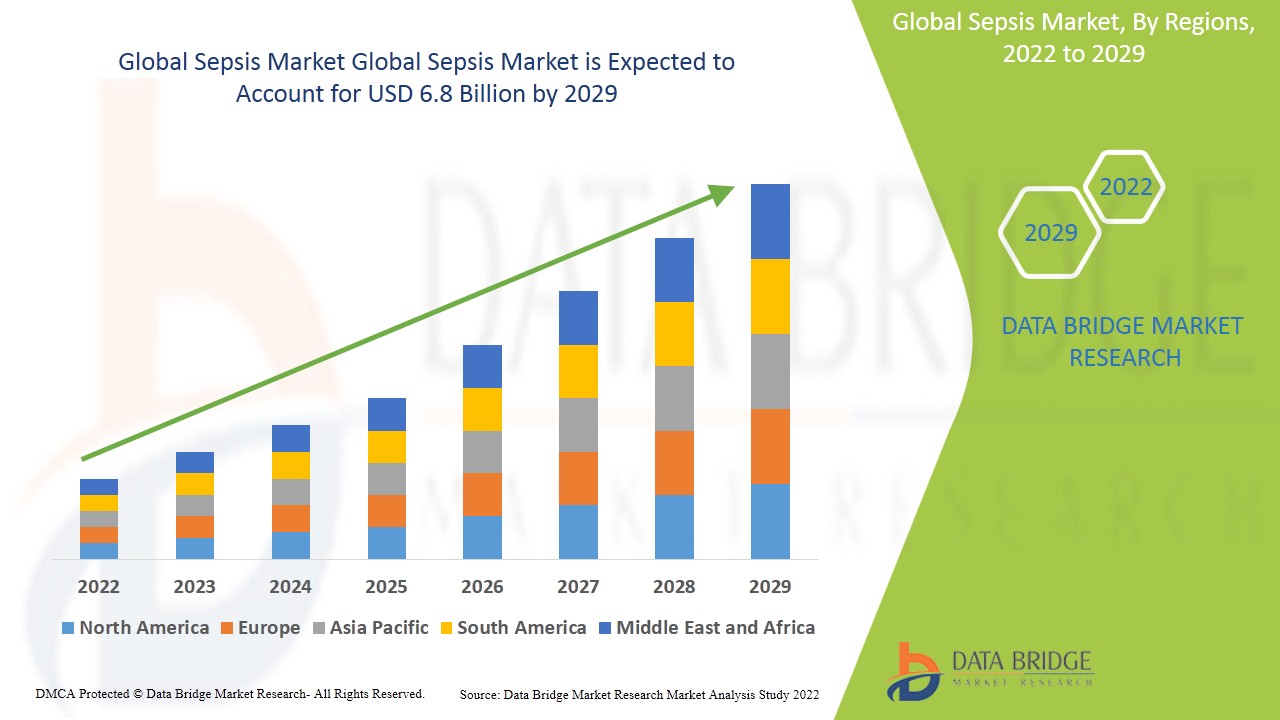
- Global sepsis market was valued at USD 3.3 billion in 2021 and is expected to reach USD 6.8 billion by 2029, registering a CAGR of 8.5% during the forecast period of 2022-2029. The “bacterial” accounts for the largest pathogen segment in the sepsis market within the forecasted period owing to the rise in the bacterial sepsis cases and the rising prevalence of HAIs. The market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Market Definition
Sepsis is a dangerous consequence that results from a weakened immune system, making patients vulnerable to bacterial, viral, fungal, parasitic, and other illnesses. It's one of the leading causes of mortality in hospitals. When the host's immune system responds to an infection, chemicals are produced by the immune system to treat the infection, resulting in sepsis. As it enters the bloodstream, it induces inflammation throughout the body. It further leads to the blood vessel leakage, blood clots, and poor blood flow, depriving the body's critical organs of oxygen and nutrition.
The sepsis is also considered as a three-staged illness that generally starts with sepsis, then progresses to severe sepsis, and lastly to septic shock, which is a medical emergency. People with a weakened immune system, youngsters, the elderly, and those suffering from chronic illnesses including diabetes, AIDS, cancer and others are at a higher risk of acquiring sepsis.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Diagnosis, Therapeutics), Product (Reagents, Assay, Instruments, Software), Technology (Microbiology, PCR, Sequencing, Biomarkers), Test Type (Lab, POC), Pathogen (Bacterial, Viral, Fungal), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies), End User (Hospitals, Pathology and Reference Laboratories), Application (Sepsis, Severe Sepsis, Septic Shock) |
|
Countries Covered |
U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia- Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa |
|
Market Players Covered |
bioMérieux (France), BD (US), Danaher (US), Biosystems Inc., (US), Luminex Corporation (US), F. Hoffmann-La Roche Ltd., (Switzerland), Thermo Fisher Scientific Inc., (US), Bruker (US), Abbott (US), Immunexpress Inc. (U.S), Axis-Shield Diagnostics Ltd. UK), Quidel Corporation (US), Siemens (Germany), EKF Diagnostics (UK), Seegene Inc., (South Korea), Boditech Med Inc., (South Korea), and Alifax S.r.l. Italy (Italy), |
Sepsis Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints and challenges. All of this is discussed in detail as below:
Drivers
-
Growing Burden Of Infectious Diseases
The growing burden of infectious diseases which leads to the rising number of sepsis incidences, is the most significant factor driving the growth for this market. Growing cases of the hospital-acquired infections (HAIs) such as urinary tract infections, pneumonia and sepsis are also expected to accelerate the overall growth of the market.
Growing Investments and Advancements
An increase in funding for sepsis-related research activities combined with the rising investments in extensive research and development activities that include new blood culture approaches for identifying and treating chronic infections are also projected to cushion the growth of the market. Moreover, the technological advances in the field of molecular diagnostics, coupled with surging requirement for quick and accurate results are estimated to generate lucrative opportunities for the market. Various technological advancements have led to the emergence of novel immunological and molecular biomarkers that enable the early detection of sepsis, which will further expand the sepsis market's growth rate in the future.
Furthermore, the expanding healthcare sector along with the increasing penetration of advanced data analytics tools are also expected to fuel market growth. Moreover, the rising awareness among the patients and doctors regarding the chronic diseases and hospital acquired infections also cushions the market’s growth within the forecasted period.
Restraints/Challenges
-
High Costs of Diagnosis
On the other hand, cost of molecular diagnostic tests is very high, which is expected to obstruct market growth.
-
Dearth of Professionals
Also, the sepsis needs to be diagnosed and treated early with help of skilled healthcare professionals. The shortage of skilled professionals or lack of trained paramedics are projected to challenge the sepsis market in the forecast period of 2022-2029.
This sepsis market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the sepsis market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Post Covid-19 Impact on Sepsis Market
The sepsis market was largely industry impacted by the outbreak of COVID-19. The sepsis symptoms such as multiorgan damage emerged in approximately 2- 5% of COVID patients, that too after 8-10 days of admission. To deal with the dilemma, the Biomedical Advanced Research and Development Authority collaborated with Beckman Coulter to build a digital algorithm to detect sepsis in COVID-19 patients. The outbreak of coronavirus and the emergence of sepsis cases among covid-19 patients boosted the demand for quick diagnosis, resulting in a faster adoption of devices, reagents, and test kits for sepsis detection. Moreover, the geriatric population are vulnerable to complications such as acute respiratory distress syndrome (ARDS) that is caused by pneumonia and raises the risk of sepsis.
Recent Developments
- In June 2021, BioMérieux, a global pioneer in in vitro diagnostics, and Special Diagnostics, a business that brings new in vitro diagnostic methods to clinical microbiology, have inked a distribution agreement for a specific reveal fast AST system in Europe. The REVEAL Rapid AST system, developed by Specific Diagnostics and based on its patented metabolomic signature technology, provides actionable results for bloodstream infections (in an average of 5 hours* directly from positive blood culture), allowing either timely de-escalation to a more focused, more appropriate, and lower-cost therapy, or life-saving rapid escalation to a more effective drug when a multidrug-resistant (MDR) infection is present.
Global Sepsis Market Scope
The sepsis market is segmented on the basis of type, product, technology, test type, pathogen, distribution channel, end user and application. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Diagnosis
- Therapeutics
On the basis of type, the sepsis market is segmented into diagnosis and therapeutics.
Product
- Reagents
- Assay
- Instruments
- Software
On the basis of product, the sepsis market is segmented into reagents, assay, instruments and software.
Technology
- Microbiology
- PCR
- Sequencing
- Biomarkers
On the basis of technology, the sepsis market is segmented into microbiology, PCR, sequencing and biomarkers.
Test Type
- Lab
- POC
On the basis of test type, the sepsis market is segmented into lab and POC.
Pathogen
- Bacterial
- Viral
- Fungal
On the basis of pathogen, the sepsis market is segmented into bacterial, viral and fungal. The bacterial segment accounts for the largest share of market during the forecast period due to the rise in the bacterial sepsis cases and the rising prevalence of HAIs.
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
On the basis of distribution channel, the sepsis market is segmented into hospital pharmacies, retail pharmacies and online pharmacies.
End User
- Hospitals
- Pathology
- Reference Laboratories
On the basis of end user, the sepsis market is bifurcated into hospitals, pathology and reference laboratories.
Application
- Sepsis
- Severe Sepsis
- Septic Shock
On the basis of application, the sepsis market is bifurcated into sepsis, severe sepsis and septic shock.
Sepsis Market Regional Analysis/Insights
The sepsis market is analyzed and market size insights and trends are provided by country, type, product, technology, test type, pathogen, distribution channel, end user and application as referenced above.
The countries covered in the sepsis market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the sepsis market because of the increased per capita healthcare expenditure, high demand for better healthcare facilities, growing adoption of new and advanced diagnostic techniques and increasing incidences of chronic diseases such as cancer, diabetes leading to sepsis within the region.
Asia-Pacific is expected to witness significant growth during the forecast period of 2022 to 2029 due to the high geriatric population, increasing prevalence of cancer and diabetes in countries such of India, China, and Australia along with the developing healthcare infrastructure within the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The sepsis market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for sepsis market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the sepsis market. The data is available for historic period 2010-2020.
Competitive Landscape and Sepsis Market Share Analysis
The sepsis market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to sepsis market.
Some of the major players operating in the sepsis market are bioMérieux (France), BD (US), Danaher (US), Biosystems Inc., (US), Luminex Corporation (US), F. Hoffmann-La Roche Ltd., (Switzerland), Thermo Fisher Scientific Inc., (US), Bruker (US), Abbott (US), Immunexpress Inc. (U.S), Axis-Shield Diagnostics Ltd. UK), Quidel Corporation (US), Siemens (Germany), EKF Diagnostics (UK), Seegene Inc., (South Korea), Boditech Med Inc., (South Korea) and Alifax S.r.l. Italy (Italy), among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL SEPSIS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL SEPSIS MARKET SIZE
2.2.1.1. VENDOR POSITIONING GRID
2.2.1.2. TECHNOLOGY LIFE LINE CURVE
2.2.1.3. TRIPOD DATA VALIDATION MODEL
2.2.1.4. MARKET GUIDE
2.2.1.5. MULTIVARIATE MODELLING
2.2.1.6. TOP TO BOTTOM ANALYSIS
2.2.1.7. CHALLENGE MATRIX
2.2.1.8. APPLICATION COVERAGE GRID
2.2.1.9. STANDARDS OF MEASUREMENT
2.2.1.10. VENDOR SHARE ANALYSIS
2.2.1.11. SALES VOLUME
2.2.1.12. DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.1.13. DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL SEPSIS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 EPIDEMIOLOGY
5.1 INCIDENCE OF ALL BY GENDER
5.2 TREATMENT RATE
5.3 MORTALITY RATE
5.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
5.5 PATEINT TREATMENT SUCCESS RATES
6 PREMIUM INSIGHTS
6.1 PATENT ANALYSIS
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH HEMATOLOGISTS
6.8 INTERVIEWS WITH PHYSICIAN
6.9 OTHER KOL SNAPSHOTS
7 INDUSTRY INSIGHTS
8 REGULATORY SCENARIO
9 GLOBAL SEPSIS MARKET, BY PRODUCT
9.1 OVERVIEW
9.2 DIAGNOSIS
9.2.1.1. BY PRODUCT
9.2.1.2. INSTRUMENTS
9.2.1.2.1. MOLECULAR DIAGNOSTIC INSTRUMENT
9.2.1.2.1.1 MARKET SHARE (USD)
9.2.1.2.1.2 MARKET VOLUME (UNITS)
9.2.1.2.1.3 AVERAGE SELLING PRICE (ASP- USD)
9.2.1.2.2. IN-VITRO DIAGNOSTIC INSTRUMENT
9.2.1.2.2.1 MARKET SHARE (USD)
9.2.1.2.2.2 MARKET VOLUME (UNITS)
9.2.1.2.2.3 AVERAGE SELLING PRICE (ASP- USD)
9.2.1.2.3. POINT OF CARE TESTING INSTRUMENT
9.2.1.2.3.1 MARKET SHARE (USD)
9.2.1.2.3.2 MARKET VOLUME (UNITS)
9.2.1.2.3.3 AVERAGE SELLING PRICE (ASP- USD)
9.2.1.3. ASSAYS & REAGENTS
9.2.1.3.1. PCR-BASED KITS
9.2.1.3.1.1 MARKET SHARE (USD)
9.2.1.3.1.2 MARKET VOLUME (UNITS)
9.2.1.3.1.3 AVERAGE SELLING PRICE (ASP- USD)
9.2.1.3.2. IMMUNOASSAY-BASED KITS
9.2.1.3.2.1 MARKET SHARE (USD)
9.2.1.3.2.2 MARKET VOLUME (UNITS)
9.2.1.3.2.3 AVERAGE SELLING PRICE (ASP- USD)
9.2.1.3.3. OTHERS
9.2.1.4. BLOOD CULTURE MEDIA
9.2.1.4.1. BROTH
9.2.1.4.2. AGAR
9.2.1.4.3. OTHERS
9.2.1.5. SOFTWARE
9.2.1.6. BY METHOD
9.2.1.7. CONVENTIONAL DIAGNOSTICS
9.2.1.8. AUTOMATED DIAGNOSTICS
9.2.1.9. BY TECHNOLOGY
9.2.1.10. MICROBIOLOGY
9.2.1.11. MOLECULAR DIAGNOSTICS
9.2.1.11.1. POLYMERASE CHAIN REACTION
9.2.1.11.2. MICROARRAYS
9.2.1.11.3. PEPTIDE NUCLEIC ACID-FLUORESCENT IN SITU HYBRIDIZATION
9.2.1.11.4. SYNDROMIC PANEL-BASED TESTING
9.2.1.12. IMMUNOASSAYS
9.2.1.13. FLOW CYTOMETRY
9.2.1.14. BY USABILITY
9.2.1.15. LABORATORY TESTING
9.2.1.16. POINT-OF-CARE TESTING
9.3 THERAPEUTICS
9.3.1.1. BY TYPE
9.3.1.2. ANTIBIOTICS
9.3.1.2.1. CEFTRIAXONE
9.3.1.2.2. MEROPENEM
9.3.1.2.3. CEFTAZIDIME
9.3.1.2.4. CEFOTAXIME
9.3.1.2.5. CEFEPIME
9.3.1.2.6. PIPERACILLIN AND TAZOBACTAM
9.3.1.2.7. AMPICILLIN AND SULBACTAM
9.3.1.2.8. IMIPENEM/CILASTATIN
9.3.1.2.9. LEVOFLOXACIN
9.3.1.2.10. CLINDAMYCIN
9.3.1.3. INTRAVENOUS FLUID REPLACEMENT
9.3.1.4. INSULIN
9.3.1.5. CYTOKINE ANTAGONISTS
9.3.1.5.1. INTERLEUTIN-1 RECEPTOR ANTAGONIST(IL-1RA)
9.3.1.5.2. INTERLEUKIN-10(IL-10)
9.3.1.5.3. TNF-Α,IL-1Β,IL-6
9.3.1.5.4. IL-8
9.3.1.6. PRR ANTAGONIST
9.3.1.6.1. TLRS
9.3.1.6.2. TLR1/TLR2
9.3.1.6.3. TLR2/TLR6
9.3.1.6.4. NLRS
9.3.1.6.5. OTHERS
9.3.1.7. PATHOGEN-ASSOCIATED MOLECULAR ANTAGONISTS
9.3.1.8. RECOMBINANT HUMAN APC (RHAPC)
9.3.1.8.1. DROTRECOGIN ALFA
9.3.1.8.2. XIGRIS
9.3.1.8.3. OTHERS
9.3.1.9. RECOMBINANT HUMAN SOLUBLE THROMBOSIS REGULATORS
9.3.1.10. PENTOXIFYLLINE
9.3.1.11. CO-INHIBITING MOLECULAR INHIBITOR
9.3.1.11.1. CYTOTOXIC T LYMPHOCYTE ANTIGEN 4
9.3.1.11.2. T-CELL IMMUNOGLOBULIN AND MUCIN DOMAIN PROTEIN 3
9.3.1.11.3. OTHERS
9.3.1.12. OTHERS
9.3.1.13. BY ROUTE OF ADMINISTRATION
9.3.1.14. INTRAVENOUS
9.3.1.15. ORAL
9.3.1.16. OTHERS
10 GLOBAL SEPSIS MARKET, BY PATHOGEN
10.1 OVERVIEW
10.2 BACTERIAL SEPSIS
10.2.1.1. GRAM-NEGATIVE BACTERIAL SEPSIS
10.2.1.2. GRAM-POSITIVE BACTERIAL SEPSIS
10.3 FUNGAL SEPSIS
10.3.1.1. CANDIDIASIS
10.3.1.2. INVASIVE CANDIDIASIS
10.3.1.3. ASPERGILLUS
10.3.1.4. OTHERS
10.4 OTHERS
11 GLOBAL SEPSIS MARKET, BY DRUG TYPE
11.1 OVERVIEW
11.2 GENERIC
11.3 BRANDED
11.3.1.1. ROCEPHIN
11.3.1.2. MERREM
11.3.1.3. FORTAZ
11.3.1.4. CLAFORAN
11.3.1.5. MAXIPIME
11.3.1.6. ZOSYN
11.3.1.7. UNASYN
11.3.1.8. PRIMAXIN
11.3.1.9. LEVAQUIN
11.3.1.10. CLEOCIN
12 GLOBAL SEPSIS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 PATHOLOGY & REFERENCE LABORATORIES
12.4 AMBULATORY SURGICAL CENTERS
12.5 DIAGNOSTIC LABORATORY
12.6 OTHERS
13 GLOBAL SEPSIS MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 OVERVIEW
13.3 DIRECT TENDER
13.4 RETAIL SALES
13.4.1.1. HOSPITAL PHARMACIES
13.4.1.2. RETAIL PHARMACIES
13.4.1.3. ONLINE PHARMACIES
14 GLOBAL SEPSIS MARKET, BY REGION
14.1 GLOBAL SEPSIS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
14.2 NORTH AMERICA
14.2.1.1. U.S.
14.2.1.2. CANADA
14.2.1.3. MEXICO
14.3 EUROPE
14.3.1.1. GERMANY
14.3.1.2. FRANCE
14.3.1.3. U.K.
14.3.1.4. ITALY
14.3.1.5. SPAIN
14.3.1.6. RUSSIA
14.3.1.7. TURKEY
14.3.1.8. BELGIUM
14.3.1.9. NETHERLANDS
14.3.1.10. SWITZERLAND
14.3.1.11. REST OF EUROPE
14.4 ASIA-PACIFIC
14.4.1.1. JAPAN
14.4.1.2. CHINA
14.4.1.3. SOUTH KOREA
14.4.1.4. INDIA
14.4.1.5. AUSTRALIA
14.4.1.6. SINGAPORE
14.4.1.7. THAILAND
14.4.1.8. MALAYSIA
14.4.1.9. INDONESIA
14.4.1.10. PHILIPPINES
14.4.1.11. REST OF ASIA-PACIFIC
14.5 SOUTH AMERICA
14.5.1.1. BRAZIL
14.5.1.2. ARGENTINA
14.5.1.3. REST OF SOUTH AMERICA
14.6 MIDDLE EAST AND AFRICA
14.6.1.1. SOUTH AFRICA
14.6.1.2. SAUDI ARABIA
14.6.1.3. UAE
14.6.1.4. EGYPT
14.6.1.5. ISRAEL
14.6.1.6. REST OF MIDDLE EAST AND AFRICA
14.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
15 GLOBAL SEPSIS MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: GLOBAL
15.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
15.3 COMPANY SHARE ANALYSIS: EUROPE
15.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15.5 MERGERS & ACQUISITIONS
15.6 NEW PRODUCT DEVELOPMENT & APPROVALS
15.7 EXPANSIONS
15.8 REGULATORY CHANGES
15.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
16 GLOBAL SEPSIS MARKET, SWOT AND DBMR ANALYSIS
17 GLOBAL SEPSIS MARKET, COMPANY PROFILE
17.1 ABBOTT
17.1.1.1. COMPANY OVERVIEW
17.1.1.2. REVENUE ANALYSIS
17.1.1.3. GEOGRAPHIC PRESENCE
17.1.1.4. PRODUCT PORTFOLIO
17.1.1.5. RECENT DEVELOPMENTS
17.2 BECKMAN COULTER (DANAHER)
17.2.1.1. COMPANY OVERVIEW
17.2.1.2. REVENUE ANALYSIS
17.2.1.3. GEOGRAPHIC PRESENCE
17.2.1.4. PRODUCT PORTFOLIO
17.2.1.5. RECENT DEVELOPMENTS
17.3 BD
17.3.1.1. COMPANY OVERVIEW
17.3.1.2. REVENUE ANALYSIS
17.3.1.3. GEOGRAPHIC PRESENCE
17.3.1.4. PRODUCT PORTFOLIO
17.3.1.5. RECENT DEVELOPMENTS
17.4 BIOMÉRIEUX
17.4.1.1. COMPANY OVERVIEW
17.4.1.2. REVENUE ANALYSIS
17.4.1.3. GEOGRAPHIC PRESENCE
17.4.1.4. PRODUCT PORTFOLIO
17.5 BIO-RAD LABORATORIES
17.5.1.1. COMPANY OVERVIEW
17.5.1.2. REVENUE ANALYSIS
17.5.1.3. GEOGRAPHIC PRESENCE
17.5.1.4. PRODUCT PORTFOLIO
17.5.1.5. RECENT DEVELOPMENTS
17.6 BRUKER CORPORATION
17.6.1.1. COMPANY OVERVIEW
17.6.1.2. REVENUE ANALYSIS
17.6.1.3. GEOGRAPHIC PRESENCE
17.6.1.4. PRODUCT PORTFOLIO
17.6.1.5. RECENT DEVELOPMENTS
17.7 CEPHEID
17.7.1.1. COMPANY OVERVIEW
17.7.1.2. REVENUE ANALYSIS
17.7.1.3. GEOGRAPHIC PRESENCE
17.7.1.4. PRODUCT PORTFOLIO
17.7.1.5. RECENT DEVELOPMENTS
17.8 CHEMBIO DIAGNOSTIC SYSTEMS
17.8.1.1. COMPANY OVERVIEW
17.8.1.2. REVENUE ANALYSIS
17.8.1.3. GEOGRAPHIC PRESENCE
17.8.1.4. PRODUCT PORTFOLIO
17.8.1.5. RECENT DEVELOPMENTS
17.9 NANOSPHERE, INC.
17.9.1.1. COMPANY OVERVIEW
17.9.1.2. REVENUE ANALYSIS
17.9.1.3. GEOGRAPHIC PRESENCE
17.9.1.4. PRODUCT PORTFOLIO
17.9.1.5. RECENT DEVELOPMENTS
17.1 ORTHO-CLINICAL DIAGNOSTICS
17.10.1.1. COMPANY OVERVIEW
17.10.1.2. REVENUE ANALYSIS
17.10.1.3. GEOGRAPHIC PRESENCE
17.10.1.4. PRODUCT PORTFOLIO
17.10.1.5. RECENT DEVELOPMENTS
17.11 F. HOFFMANN-LA ROCHE LTD
17.11.1.1. COMPANY OVERVIEW
17.11.1.2. REVENUE ANALYSIS
17.11.1.3. GEOGRAPHIC PRESENCE
17.11.1.4. PRODUCT PORTFOLIO
17.11.1.5. RECENT DEVELOPMENTS
17.12 SIEMENS HEALTHCARE GMBH
17.12.1.1. COMPANY OVERVIEW
17.12.1.2. REVENUE ANALYSIS
17.12.1.3. GEOGRAPHIC PRESENCE
17.12.1.4. PRODUCT PORTFOLIO
17.12.1.5. RECENT DEVELOPMENTS
17.13 T2 BIOSYSTEMS, INC.
17.13.1.1. COMPANY OVERVIEW
17.13.1.2. REVENUE ANALYSIS
17.13.1.3. GEOGRAPHIC PRESENCE
17.13.1.4. PRODUCT PORTFOLIO
17.13.1.5. RECENT DEVELOPMENTS
17.14 THERMO FISHER SCIENTIFIC INC.
17.14.1.1. COMPANY OVERVIEW
17.14.1.2. REVENUE ANALYSIS
17.14.1.3. GEOGRAPHIC PRESENCE
17.14.1.4. PRODUCT PORTFOLIO
17.14.1.5. RECENT DEVELOPMENTS
17.15 LUMINEX CORPORATION
17.15.1.1. COMPANY OVERVIEW
17.15.1.2. REVENUE ANALYSIS
17.15.1.3. GEOGRAPHIC PRESENCE
17.15.1.4. PRODUCT PORTFOLIO
17.15.1.5. RECENT DEVELOPMENTS
17.16 IMMUNEXPRESS INC
17.16.1.1. COMPANY OVERVIEW
17.16.1.2. REVENUE ANALYSIS
17.16.1.3. GEOGRAPHIC PRESENCE
17.16.1.4. PRODUCT PORTFOLIO
17.16.1.5. RECENT DEVELOPMENTS
17.17 GENTIAN DIAGNOSTICS AS
17.17.1.1. COMPANY OVERVIEW
17.17.1.2. REVENUE ANALYSIS
17.17.1.3. GEOGRAPHIC PRESENCE
17.17.1.4. PRODUCT PORTFOLIO
17.17.1.5. RECENT DEVELOPMENTS
17.18 CALA MEDICAL
17.18.1.1. COMPANY OVERVIEW
17.18.1.2. REVENUE ANALYSIS
17.18.1.3. GEOGRAPHIC PRESENCE
17.18.1.4. PRODUCT PORTFOLIO
17.18.1.5. RECENT DEVELOPMENTS
17.19 ALIFAX S.R.L.
17.19.1.1. COMPANY OVERVIEW
17.19.1.2. REVENUE ANALYSIS
17.19.1.3. GEOGRAPHIC PRESENCE
17.19.1.4. PRODUCT PORTFOLIO
17.19.1.5. RECENT DEVELOPMENTS
17.2 ALPHA LABORATORIES
17.20.1.1. COMPANY OVERVIEW
17.20.1.2. REVENUE ANALYSIS
17.20.1.3. GEOGRAPHIC PRESENCE
17.20.1.4. PRODUCT PORTFOLIO
17.20.1.5. RECENT DEVELOPMENTS
17.21 BODITECH MED
17.21.1.1. COMPANY OVERVIEW
17.21.1.2. REVENUE ANALYSIS
17.21.1.3. GEOGRAPHIC PRESENCE
17.21.1.4. PRODUCT PORTFOLIO
17.21.1.5. RECENT DEVELOPMENTS
17.22 GENMARK DIAGNOSTICS
17.22.1.1. COMPANY OVERVIEW
17.22.1.2. REVENUE ANALYSIS
17.22.1.3. GEOGRAPHIC PRESENCE
17.22.1.4. PRODUCT PORTFOLIO
17.22.1.5. RECENT DEVELOPMENTS
17.23 SEEGENE
17.23.1.1. COMPANY OVERVIEW
17.23.1.2. REVENUE ANALYSIS
17.23.1.3. GEOGRAPHIC PRESENCE
17.23.1.4. PRODUCT PORTFOLIO
17.23.1.5. RECENT DEVELOPMENTS
17.24 EKF DIAGNOSTICS
17.24.1.1. COMPANY OVERVIEW
17.24.1.2. REVENUE ANALYSIS
17.24.1.3. GEOGRAPHIC PRESENCE
17.24.1.4. PRODUCT PORTFOLIO
17.24.1.5. RECENT DEVELOPMENTS
17.25 AXIS-SHIELD DIAGNOSTICS
17.25.1.1. COMPANY OVERVIEW
17.25.1.2. REVENUE ANALYSIS
17.25.1.3. GEOGRAPHIC PRESENCE
17.25.1.4. PRODUCT PORTFOLIO
17.25.1.5. RECENT DEVELOPMENTS
17.26 TRINITY BIOTECH
17.26.1.1. COMPANY OVERVIEW
17.26.1.2. REVENUE ANALYSIS
17.26.1.3. GEOGRAPHIC PRESENCE
17.26.1.4. PRODUCT PORTFOLIO
17.26.1.5. RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
18 RELATED REPORTS
19 QUESTIONNAIRE
20 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.