Global Spinocerebellar Ataxias Scas Market
Market Size in USD Million
CAGR :
% 
 USD
373.74 Million
USD
523.41 Million
2024
2032
USD
373.74 Million
USD
523.41 Million
2024
2032
| 2025 –2032 | |
| USD 373.74 Million | |
| USD 523.41 Million | |
|
|
|
|
Spinocerebellar Ataxias (SCA’s) Market Size
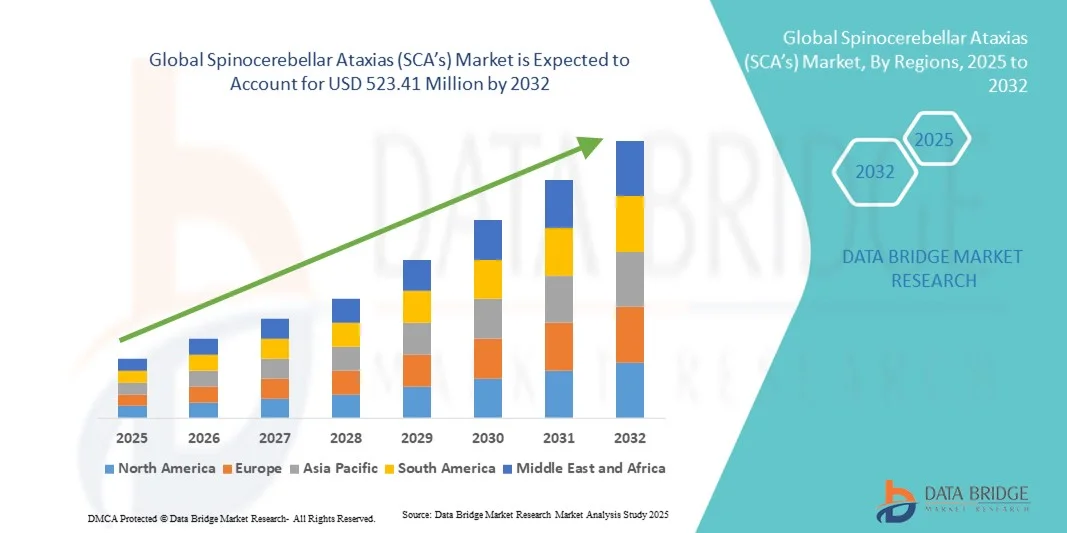
- The global spinocerebellar ataxias (SCA’s) market size was valued at USD 373.74 Million in 2024 and is expected to reach USD 523.41 Million by 2032, at a CAGR of 4.30% during the forecast period
- The market growth is largely fueled by the increasing prevalence of Spinocerebellar Ataxias (SCA’s) globally, coupled with rising awareness about early diagnosis and management options among patients and healthcare providers
- Furthermore, growing investments in research and development for novel therapies, along with advancements in genetic testing and precision medicine, are accelerating the uptake of Spinocerebellar Ataxias (SCA’s) solutions, thereby significantly boosting the industry's growth
Spinocerebellar Ataxias (SCA’s) Market Analysis
- Spinocerebellar Ataxias (SCA’s), a group of hereditary and sporadic neurodegenerative disorders, are increasingly critical in clinical neurology due to their progressive impact on motor coordination and quality of life
- The escalating demand for spinocerebellar ataxias (SCA’s) treatments and diagnostics is primarily fueled by growing awareness among healthcare providers, advancements in genetic testing, and the rising preference for personalized medicine approaches
- North America dominated the spinocerebellar ataxias (SCA’s) market with the largest revenue share of 42.5% in 2024, characterized by advanced healthcare infrastructure, high healthcare expenditure, and a strong presence of key pharmaceutical and biotechnology players, with the U.S. experiencing substantial growth in spinocerebellar ataxias (SCA’s) diagnostics and therapies, driven by innovations in gene therapy and precision medicine
- Asia-Pacific is expected to be the fastest growing region in the spinocerebellar ataxias (SCA’s) market during the forecast period, due to increasing healthcare access, rising awareness of neurological disorders, and expanding diagnostic and treatment facilities
- The adults segment accounted for the largest market revenue share of 65% in 2024, driven by the higher prevalence of late-onset SCA subtypes and a growing elderly population
Report Scope and Spinocerebellar Ataxias (SCA’s) Market Segmentation
|
Attributes |
Spinocerebellar Ataxias (SCA’s) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Spinocerebellar Ataxias (SCA’s) Market Trends
Enhanced Convenience Through AI and Digital Health Integration
- A significant and accelerating trend in the global spinocerebellar ataxias (SCA’s) market is the deepening integration of artificial intelligence (AI) and digital health platforms into patient management and therapeutic monitoring. This combination of technologies is significantly enhancing patient convenience, improving disease monitoring, and supporting personalized care approaches
- For instance, AI-powered SCA management platforms can track motor function progression, analyze patient gait patterns, and provide real-time alerts to clinicians regarding changes in disease status. Similarly, digital health applications for SCA patients enable remote symptom tracking, medication adherence monitoring, and teleconsultation integration, offering a comprehensive disease management solution
- AI integration in SCA management enables predictive analytics for disease progression, identification of early symptoms, and the optimization of therapeutic interventions. For example, certain digital platforms can analyze longitudinal patient data to suggest adjustments in physical therapy regimens or potential pharmacological interventions. These capabilities allow for proactive management strategies, potentially improving patient outcomes and quality of life
- The seamless integration of AI-based SCA monitoring tools with digital health platforms facilitates centralized management of patient data. Through a single interface, healthcare providers can monitor motor function, cognitive symptoms, and treatment adherence while coordinating interventions and support across multidisciplinary care teams. This approach creates a unified, patient-centered healthcare experience that enhances both efficiency and clinical decision-making
- This trend towards more intelligent, data-driven, and interconnected SCA management systems is fundamentally reshaping expectations among clinicians, patients, and caregivers. Consequently, companies such as WECARE and NeuroTrack are developing AI-enabled SCA platforms with features such as automatic symptom trend detection, predictive risk alerts, and integration with remote care platforms
- The demand for AI-enabled and digitally integrated SCA solutions is growing rapidly across clinical and research settings, as patients and healthcare providers increasingly prioritize convenience, proactive disease monitoring, and comprehensive digital health functionality
Spinocerebellar Ataxias (SCA’s) Market Dynamics
Driver
Growing Need Due to Rising Disease Awareness and Digital Health Adoption
- The increasing prevalence of spinocerebellar Ataxias and rising awareness of early diagnosis and disease monitoring, coupled with the accelerating adoption of digital health ecosystems, is a significant driver for the heightened demand for AI-based SCA solutions
- For instance, in April 2024, announced advancements in AI-powered neurological assessment platforms, with plans to integrate state-of-the-art predictive analytics into its SCA monitoring suite. Such strategies by key companies are expected to drive growth in the SCA market during the forecast period
- As patients and clinicians become more aware of potential disease progression and complications, AI-enabled SCA management tools offer advanced features such as symptom trend analysis, personalized intervention recommendations, and real-time monitoring alerts, providing a compelling improvement over conventional care approaches
- Furthermore, the growing popularity of digital health devices and the desire for interconnected patient management systems are making AI-driven SCA solutions an integral component of modern healthcare, enabling seamless integration with electronic health records, telemedicine platforms, and rehabilitation programs
- The convenience of remote monitoring, automated symptom tracking, and data-driven insights for both clinicians and patients are key factors propelling the adoption of AI-enabled SCA solutions in clinical and research settings. The trend toward user-friendly platforms and patient-centric digital tools further contributes to market growth
Restraint/Challenge
Concerns Regarding Data Security and High Implementation Costs
- Concerns surrounding the cybersecurity and privacy of patient data in AI-driven SCA solutions pose a significant challenge to broader market penetration. As digital platforms rely on cloud connectivity and sophisticated software, they are susceptible to data breaches and unauthorized access, raising anxieties among potential users about the confidentiality of sensitive medical information
- For instance, high-profile reports of vulnerabilities in healthcare IoT devices and digital platforms have made some patients and providers cautious about adopting AI-enabled disease management solutions, including SCA monitoring tools
- Addressing these cybersecurity concerns through robust encryption, secure authentication protocols, and regular software updates is crucial for building trust. Companies such as NeuroTrack and WECARE emphasize advanced security measures in their solutions to reassure patients and clinicians. In addition, the relatively high initial cost of implementing comprehensive AI-based SCA monitoring platforms compared to traditional clinical tools can be a barrier to adoption, particularly in emerging regions or for resource-constrained healthcare facilities
- While costs are gradually decreasing, the perceived premium for AI and digital health solutions can still hinder widespread adoption, especially for those who do not immediately recognize the long-term clinical and operational benefits
- Overcoming these challenges through enhanced cybersecurity, clinician and patient education, and the development of cost-effective AI-enabled SCA tools will be vital for sustained market growth in the forecast period
Spinocerebellar Ataxias (SCA’s) Market Scope
The market is segmented on the basis of type, diagnosis, treatment, population type, end-user, and distribution channel.
- By Type
On the basis of type, the Spinocerebellar Ataxias (SCA’s) market is segmented into SCA1, SCA2, SCA3, SCA6, and others. The SCA3 segment dominated the largest market revenue share of 38.5% in 2024, driven by its higher prevalence globally and extensive research focus on neurodegenerative progression. The segment benefits from well-established clinical awareness, availability of specialized diagnostic protocols, and active patient registries that facilitate early detection and treatment. Pharmaceutical and biotechnology companies are prioritizing SCA3 for clinical trials and therapeutic development due to its significant impact on motor coordination and patient quality of life. Increasing government and non-profit funding for rare neurodegenerative disorders further strengthens the segment. Advanced imaging and genetic profiling techniques are increasingly applied to monitor disease progression, enabling more personalized care and management. Hospitals and specialty clinics show a preference for SCA3-focused interventions because of robust treatment guidelines and evidence-based protocols. This dominance is reinforced by collaborations between research institutes, patient advocacy groups, and pharmaceutical developers. The segment also sees continuous innovation in symptom management therapies, adaptive devices, and rehabilitation solutions. Multidisciplinary care approaches, including physiotherapy and occupational therapy, contribute to better outcomes for SCA3 patients. Awareness programs for neurologists and caregivers have enhanced early detection rates, further sustaining market share.
The SCA6 segment is anticipated to witness the fastest growth rate of 22.1% CAGR from 2025 to 2032, driven by increasing identification of late-onset cases and rising genetic testing adoption. Growing investments in rare disease research and off-label therapy development bolster this segment’s expansion. Innovative therapeutic interventions targeting cerebellar degeneration are under development, enhancing clinical management. The segment also benefits from increased accessibility of specialized neurology clinics in emerging markets and improved patient registries. Awareness campaigns by patient advocacy groups are accelerating diagnosis and early intervention. Pharmaceutical companies are focusing on SCA6 for pipeline expansion, with several preclinical studies showing promising results. The integration of digital health tools for remote monitoring and disease tracking contributes to the growth of this segment. Expansion of genetic counseling services further supports early detection and patient management. Clinical collaborations with international research centers enhance data collection, enabling more efficient therapy design. The growing adoption of adaptive devices and supportive therapies also drives demand. Regional healthcare policies supporting rare disease care enhance access to SCA6-focused treatment options. Increased publication of scientific research and case studies encourages further innovation. Overall, these factors position SCA6 as the fastest-growing subtype in the market.
- By Diagnosis
On the basis of diagnosis, the Spinocerebellar Ataxias (SCA’s) market is segmented into imaging tests, lumbar puncture tests, and genetic tests. The genetic tests segment held the largest revenue share of 45% in 2024, owing to their precision in identifying specific SCA mutations and providing definitive confirmation. Genetic testing enables early diagnosis, carrier detection, and family planning, which is critical in hereditary disorders like SCA. The widespread adoption of next-generation sequencing (NGS) and polymerase chain reaction (PCR) techniques has enhanced accuracy and reduced turnaround time. Major hospitals and specialty clinics prefer genetic tests for their reliability and ability to inform personalized treatment plans. Increasing awareness among neurologists and patients regarding hereditary neurodegenerative disorders drives demand. Government support for rare disease diagnostics, reimbursement policies, and inclusion in clinical guidelines further strengthen market penetration. Collaboration between diagnostic labs and research institutions facilitates the development of mutation-specific panels. Genetic counseling is often coupled with testing, improving patient outcomes and adherence to treatment. Commercial partnerships between biotech firms and diagnostics companies expand access to testing in emerging regions. Continuous technological advancements and cost reduction in genetic testing contribute to sustained market dominance.
The imaging tests segment is expected to witness the fastest CAGR of 20.5% from 2025 to 2032, driven by advances in MRI, fMRI, and diffusion tensor imaging technologies. These tools allow early detection of cerebellar atrophy and disease progression monitoring, facilitating clinical decision-making. Increased investments in neuroimaging infrastructure by hospitals and specialty clinics enhance adoption. Imaging tests are particularly critical for research studies and longitudinal patient tracking. Growth is supported by collaborations between radiology departments and neurology centers to integrate imaging with genetic data. Development of portable and high-resolution imaging devices improves accessibility in regional and remote healthcare centers. The integration of AI-based image analysis tools accelerates diagnosis and provides quantitative biomarkers for treatment evaluation. Educational initiatives and clinical workshops raise awareness among neurologists about imaging utility in SCA. Funding from rare disease foundations encourages imaging studies in underrepresented populations. Emerging markets are increasingly adopting imaging diagnostics due to decreasing equipment costs. Insurance coverage expansion for neuroimaging procedures also contributes to segment growth.
- By Treatment
On the basis of treatment, the Spinocerebellar Ataxias (SCA’s) market is segmented into adaptive devices, therapies, and off-label treatments. The therapies segment dominated the largest market revenue share of 52% in 2024, owing to the broad range of rehabilitation, physical therapy, and occupational therapy interventions that improve patient mobility and quality of life. Multidisciplinary therapeutic programs are widely implemented in hospitals and specialty clinics. Advanced neurorehabilitation devices and protocol-driven therapies enhance motor function and delay disease progression. Therapy adoption is reinforced by clinical guidelines, insurance coverage, and increasing patient awareness. Pharmaceutical research targeting symptom relief also complements therapy services. Collaboration between academic centers and therapy providers enhances innovation and standardization of treatment protocols. Tele-rehabilitation solutions are emerging to improve access in remote regions. Patient support programs and caregiver training further strengthen adoption. Continuous publication of clinical outcomes validates therapeutic effectiveness, reinforcing the segment’s dominance.
The off-label treatments segment is anticipated to witness the fastest CAGR of 19.8% from 2025 to 2032, fueled by ongoing research into repurposed drugs and small-molecule therapeutics. Pharmaceutical companies are exploring off-label options to manage ataxia symptoms, with several clinical trials underway. Growth is further supported by increasing patient enrollment in global studies. Emerging markets show rising adoption due to cost-effective access to off-label medications. Integration of off-label therapies with rehabilitation programs enhances efficacy. Personalized medicine approaches and biomarker-driven interventions drive uptake. Collaboration with international rare disease networks facilitates knowledge transfer. Education and awareness campaigns promote acceptance among clinicians. Continuous regulatory guidance on compassionate use programs encourages adoption. Technological platforms for monitoring therapeutic outcomes contribute to market expansion.
- By Population Type
On the basis of population type, the Spinocerebellar Ataxias (SCA’s) market is segmented into children and adults. The adults segment accounted for the largest market revenue share of 65% in 2024, driven by the higher prevalence of late-onset SCA subtypes and a growing elderly population. Adults are more likely to seek specialized neurological care, undergo diagnostic testing, and enroll in clinical trials. Healthcare infrastructure and insurance coverage for adult neurology care enhance market adoption. Adult-focused research studies and patient registries facilitate disease management and therapy development. Awareness campaigns targeting adult patients support early detection. Multidisciplinary care and long-term monitoring programs further strengthen market dominance.
The children segment is expected to witness the fastest CAGR of 21.2% from 2025 to 2032, driven by early genetic screening programs, pediatric neurology investments, and increasing availability of adaptive devices and therapies for pediatric patients. Growth is fueled by rare disease advocacy and inclusion of children in clinical studies. Specialized pediatric clinics and research initiatives expand treatment access. Integration of telehealth solutions allows remote monitoring and therapy delivery. Early intervention strategies improve long-term outcomes and reduce disease progression. Educational programs for parents and caregivers support adherence to therapy regimens. Funding from government and non-profit organizations promotes research in pediatric SCA management. Multidisciplinary pediatric care centers contribute to faster adoption of diagnostic and therapeutic solutions.
- By End-User
On the basis of end-user, the Spinocerebellar Ataxias (SCA’s) market is segmented into hospitals, specialty clinics, and others. The hospitals segment dominated the largest revenue share of 55% in 2024, driven by advanced diagnostic infrastructure, multidisciplinary neurology departments, and comprehensive therapy programs. Hospitals provide both clinical trials and routine patient care, reinforcing market dominance. Strong hospital networks in developed regions facilitate patient access to treatments. Hospitals also serve as hubs for patient education and genetic counseling, enhancing adoption. Established relationships with pharmaceutical and biotechnology companies allow hospitals to implement innovative therapies efficiently. Hospitals benefit from large patient volumes, enabling economies of scale in diagnostics and treatments. They are equipped with state-of-the-art imaging technologies and genetic testing facilities. In addition, hospitals participate in regional and global registries, improving patient monitoring. The integration of rehabilitation, speech, and occupational therapy further consolidates the segment’s leading position. Strong reimbursement policies and insurance coverage in key countries also promote hospital utilization. Regulatory approvals are often first targeted at hospital-based programs, reinforcing their primary role in the market. The segment also benefits from high awareness among neurologists and healthcare professionals about SCA management protocols.
The specialty clinics segment is anticipated to witness the fastest CAGR of 20.6% from 2025 to 2032, driven by increasing numbers of neurology-focused outpatient centers and centers of excellence. These clinics offer personalized care, early diagnosis, and specialized therapies. Rising awareness among patients and caregivers supports adoption. Specialty clinics are particularly attractive for follow-up, rehabilitation, and monitoring programs. Integration with research initiatives and clinical trials enhances credibility and uptake. Growth is fueled by partnerships with biotechnology and pharmaceutical companies for patient support and therapy evaluation. Clinics often provide flexible appointment scheduling, telemedicine services, and patient education programs, which improve treatment adherence. They focus on targeted populations, including children and adults, for specialized interventions. Investment in advanced diagnostic tools and genetic counseling services is increasing. Clinics leverage collaborations with hospitals for complex cases, expanding patient access. Enhanced patient experience and shorter wait times drive preference for specialty clinics. The development of clinic networks across urban and semi-urban areas is promoting regional expansion. Government and private funding for rare disease programs further accelerates segment growth. Rising prevalence of SCA and increased emphasis on early intervention in outpatient settings are key growth enablers.
- By Distribution Channel
On the basis of distribution channel, the Spinocerebellar Ataxias (SCA’s) market is segmented into direct tender, retail sales, and others. The direct tender segment held the largest market revenue share of 50% in 2024, driven by institutional procurement by hospitals, specialty clinics, and research organizations. Bulk purchasing agreements and government contracts enhance dominance. Direct tender ensures timely supply of diagnostics, therapies, and adaptive devices to healthcare institutions. It provides cost efficiencies due to high-volume orders and long-term contracts. Hospitals and clinics prefer direct tender for reliability, traceability, and compliance with regulatory standards. Centralized procurement reduces administrative burden and streamlines logistics. Direct tender also supports large-scale clinical trials by ensuring uninterrupted availability of therapies. The segment benefits from strategic partnerships between manufacturers and government agencies. Pricing agreements and reimbursement support further reinforce the segment’s dominance. Major suppliers maintain dedicated distribution teams for institutional clients.
The retail sales segment is expected to witness the fastest CAGR of 18.9% from 2025 to 2032, fueled by increasing home care solutions, patient access to adaptive devices, and availability of off-label therapies. Online retail platforms and pharmacy networks contribute to convenience and wider adoption. Growth is supported by rising patient awareness and demand for self-administered therapies. The expansion of e-commerce channels allows patients in remote regions to access therapies and adaptive devices. Retail distribution ensures faster replenishment of essential medications and equipment. Partnerships with specialty pharmacies enhance product availability. Convenience, accessibility, and competitive pricing drive adoption. Retail channels are increasingly integrating digital tools for patient education and adherence monitoring. Marketing campaigns and patient support programs amplify awareness. Home healthcare growth and telemedicine adoption accelerate reliance on retail channels. Rising prevalence of SCA and increasing diagnosis rates support segment growth.
Spinocerebellar Ataxias (SCA’s) Market Regional Analysis
- North America dominated the spinocerebellar ataxias (SCA’s) market with the largest revenue share of 42.5% in 2024
- Characterized by advanced healthcare infrastructure, high healthcare expenditure, and a strong presence of key pharmaceutical and biotechnology player
- The market experienced substantial growth in Spinocerebellar Ataxias (SCA’s) diagnostics and therapies, driven by innovations in gene therapy, precision medicine, and advanced neurological care
U.S. Spinocerebellar Ataxias (SCA’s) Market Insight
The U.S. spinocerebellar ataxias (SCA’s) market captured the largest revenue share of 78% in 2024 within North America, fueled by increasing investment in rare neurological disorder research, availability of cutting-edge diagnostic tools, and growing adoption of personalized treatment approaches. Expansion of clinical trials and early access programs for novel therapies further propels market growth.
Europe Spinocerebellar Ataxias (SCA’s) Market Insight
The Europe spinocerebellar ataxias (SCA’s) market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increased government funding for neurological disorder research, rising prevalence of SCAs, and growing awareness about rare neurological conditions. Key countries such as Germany, the U.K., and France are witnessing significant adoption of advanced diagnostic and therapeutic solutions across hospitals and specialty clinics.
U.K. Spinocerebellar Ataxias (SCA’s) Market Insight
The U.K. spinocerebellar ataxias (SCA’s) market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the government’s emphasis on rare disease research and funding for clinical trials. Additionally, awareness campaigns and patient support programs are encouraging early diagnosis and adoption of advanced therapies for SCA subtypes.
Germany Spinocerebellar Ataxias (SCA’s) Market Insight
The Germany spinocerebellar ataxias (SCA’s) market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing healthcare expenditure, early adoption of novel therapies, and the availability of advanced diagnostic infrastructure. Germany’s focus on research and innovation in neurodegenerative diseases promotes the growth of SCA-related healthcare solutions.
Asia-Pacific Spinocerebellar Ataxias (SCA’s) Market Insight
The Asia-Pacific spinocerebellar ataxias (SCA’s) market is poised to grow at the fastest CAGR of 24% during the forecast period of 2025 to 2032, driven by increasing healthcare access, rising awareness of neurological disorders, and expansion of diagnostic and treatment facilities in countries such as China, Japan, and India. The region’s growing neurological care infrastructure and government support for rare disease management are key growth enablers.
Japan Spinocerebellar Ataxias (SCA’s) Market Insight
The Japan spinocerebellar ataxias (SCA’s) market is gaining momentum due to the country’s advanced healthcare system, rising geriatric population, and demand for specialized neurological care. Increasing investment in genetic testing, early diagnosis, and personalized therapy solutions for SCA subtypes is driving market growth.
China Spinocerebellar Ataxias (SCA’s) Market Insight
The China spinocerebellar ataxias (SCA’s) market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to expanding healthcare access, growing awareness about rare neurological disorders, and increasing investments in diagnostic and therapeutic infrastructure. China’s focus on precision medicine and the development of specialized neurological centers supports rapid adoption of advanced SCA solutions.
Spinocerebellar Ataxias (SCA’s) Market Share
The Spinocerebellar Ataxias (SCA’s) industry is primarily led by well-established companies, including:
- Biogen (U.S.)
- Ionis Pharmaceuticals (U.S.)
- Novartis AG(Switzerland)
- Roche (Switzerland)
- PTC Therapeutics (U.S.)
- Wave Life Sciences (U.S.)
- Sanofi (France)
- Mitsubishi Tanabe Pharma (Japan)
- Orchard Therapeutics (U.K.)
Latest Developments in Global Spinocerebellar Ataxias (SCA’s) Market
- In February 2025, Biohaven Ltd., a leading biopharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) accepted its New Drug Application (NDA) for Troriluzole (BHV-4157), granting Priority Review for the treatment of adult patients with Spinocerebellar Ataxia (SCA). Troriluzole has demonstrated promising Phase 3 clinical trial results, showing a significant slowing of disease progression over a three-year period. The Priority Review designation underscores Biohaven’s commitment to delivering innovative therapies for neurodegenerative disorders and accelerating access to potentially transformative treatments for patients with SCA
- In March 2025, Kissei Pharmaceutical Co., Ltd., a Japan-based pharmaceutical company, announced the initiation of an additional Phase III clinical trial for Rovatirelin (KPS-0373) for the treatment of spinocerebellar degeneration. This follows the earlier withdrawal of a marketing application in July 2023 due to insufficient clinical data. The new trial aims to provide robust evidence of Rovatirelin’s efficacy in improving ataxia symptoms and demonstrates Kissei’s ongoing dedication to advancing therapeutic options for SCA patients
- In April 2023, Vico Therapeutics, a biotechnology company specializing in gene silencing therapies, announced the dosing of the first patient in a Phase 1/2a clinical trial of VO659 targeting Huntington’s Disease and Spinocerebellar Ataxia Types 1 and 3. This milestone represents a significant advancement in the exploration of innovative gene therapies for neurodegenerative disorders and highlights Vico Therapeutics’ focus on addressing unmet medical needs in SCA
- In September 2024, Biohaven Ltd. reported positive topline results from its pivotal study of Troriluzole in multiple SCA types, including types 1, 2, 3, 6, 7, 8, and 10. Patients participating in the trial demonstrated meaningful improvements in both symptomatic and functional outcomes. These findings reinforce Troriluzole’s potential as a novel therapeutic option for the SCA patient population and reflect Biohaven’s commitment to advancing research in rare neurodegenerative diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.