Global Szary Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
806.13 Million
USD
1,094.79 Million
2024
2032
USD
806.13 Million
USD
1,094.79 Million
2024
2032
| 2025 –2032 | |
| USD 806.13 Million | |
| USD 1,094.79 Million | |
|
|
|
|
Sézary Syndrome Market Size
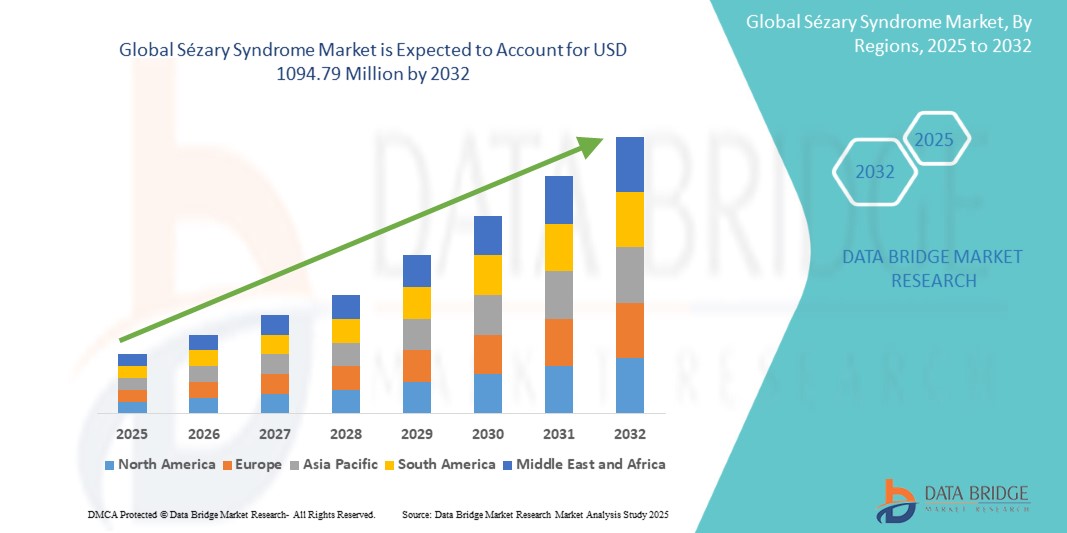
- The global sézary syndrome market size was valued at USD 806.13 million in 2024 and is expected to reach USD 1094.79 million by 2032, at a CAGR of 3.90% during the forecast period
- This growth is driven by increasing awareness of cutaneous T-cell lymphomas, advancements in targeted therapies, and rising availability of diagnostic tools.
Sézary Syndrome Market Analysis
- Sézary syndrome is an aggressive form of cutaneous T-cell lymphoma characterized by erythroderma, lymphadenopathy, and malignant T-cells in blood. It requires systemic and often long-term management using targeted immunotherapies and supportive care
- The market is expanding due to heightened diagnostic awareness, especially through flow cytometry and skin biopsies, and improved access to novel therapies including HDAC inhibitors and monoclonal antibodies
- North America is expected to dominate the sézary syndrome market with a share of 42.7%, driven by advanced diagnostic capabilities, higher disease awareness, and early access to FDA-approved therapies
- Asia-Pacific is projected to be the fastest growing region during the forecast period due to increasing incidence reporting, improved access to specialized cancer centers, and introduction of biosimilars
- In 2025, the immunotherapy segment is projected to dominate the market with market share of 39.6% due to its high efficacy, lower systemic toxicity compared to chemotherapy, and its ability to offer long-lasting immune response in various cancers and immune-related disorders
Report Scope and Sézary Syndrome Market Segmentation
|
Attributes |
Sézary Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Sézary Syndrome Market Trends
"Adoption of Targeted and Epigenetic Therapies"
- One significant trend in the Sézary Syndrome market is the increasing adoption of targeted agents and epigenetic modulators, which show promise in difficult-to-treat CTCL subsets
- The need for long-term disease control and reduced toxicity is driving interest in HDAC inhibitors and monoclonal antibodies with selective activity
For instance, recent studies have highlighted the improved progression-free survival achieved with brentuximab vedotin in patients with CD30-positive variants of Sézary Syndrome
- This shift is transforming the treatment landscape toward precision oncology, with focus on patient genotype and disease stage
- Advancements in companion diagnostics and treatment monitoring tools are further supporting the trend of early and tailored therapeutic intervention
A key trend is increased investment in next-generation agents that target genetic drivers of T-cell dysregulation in Sézary Syndrome. Clinical trials on dual-action HDAC and immune checkpoint inhibitors are ongoing
Sézary Syndrome Market Dynamics
Driver
"Expansion of Orphan Drug Approvals and Reimbursement Incentives"
- Governments and regulatory agencies are prioritizing rare disease drug development with incentives like orphan drug designations, fast-track approvals, and funding support
- These policies are encouraging companies to develop therapies for Sézary Syndrome, a rare CTCL subtype, and accelerating patient access
- Reimbursement systems are also evolving to cover high-cost specialty therapies that show meaningful clinical benefit
For instance, FDA's breakthrough therapy designation for mogamulizumab has facilitated faster development and coverage adoption in multiple markets
- Continued policy support is expected to increase investment and global availability of therapies targeting Sézary Syndrom
Opportunity
"Strategic Research Collaborations and Biotech Partnerships"
- Pharmaceutical and biotech companies are forming partnerships with academic institutions and oncology networks to advance drug discovery for Sézary Syndrome
- These collaborations are accelerating translational research and the development of biomarker-driven therapies
- Joint ventures also expand access to patient populations for clinical trials and real-world evidence collection
For instance, collaborations between European CTCL registries and U.S. cancer centers have improved trial enrollment and global data sharing
- Strategic partnerships are expected to enhance innovation and market reach across multiple therapeutic categories
Restraint/Challenge
"High Therapy Costs and Access Disparities"
- Advanced biologics and targeted therapies for Sézary Syndrome often involve high treatment costs and long administration cycles, limiting access in low-resource settings
- Inequities in healthcare funding and lack of rare disease infrastructure exacerbate access challenges, especially in rural or underserved regions
- For instance, in many emerging markets, therapies like romidepsin remain unavailable or unaffordable despite clinical need
- Cost-effectiveness remains a barrier to widespread adoption of novel treatments, particularly in non-urban cancer centers
Sézary Syndrome Market Scope
The market is segmented on the basis of drug class, route of administration, indication, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Treatment Type |
|
|
By Diagnosis |
|
|
By Route of Administration |
|
|
By Drug Class |
|
|
By Therapy Type |
|
|
By End Users |
|
|
By Distribution Channel |
|
In 2025, the Immunotherapy is projected to dominate the market with a largest share in type segment
In 2025, the immunotherapy segment is projected to dominate the market with market share of 39.6% due to its high efficacy, lower systemic toxicity compared to chemotherapy, and its ability to offer long-lasting immune response in various cancers and immune-related disorders. Increasing approvals of checkpoint inhibitors and CAR-T cell therapies, along with rising investments in immuno-oncology research, are further fueling this segment. In addition, growing awareness among healthcare providers and patients regarding personalized treatment options is boosting the adoption of immunotherapy across key markets.
The hospitals is expected to account for the largest share during the forecast period in product segment
The hospitals segment is expected to hold the largest share of around 45.2% in the end-user category during the forecast period. Hospitals remain the primary point of care for complex diseases, offering access to advanced diagnostics, multi-specialty treatment options, and inpatient services. Their capability to administer high-cost therapies such as biologics and immunotherapies under medical supervision enhances their dominance. Moreover, government funding, expansion of tertiary care centers, and integrated pharmacy services are contributing to the strong positioning of hospitals in the treatment landscape.
Sézary Syndrome Market Regional Analysis
“North America Holds the Largest Share in the Sézary Syndrome Market”
- North America dominates the Sézary Syndrome market with a share of 42.7%, attributed to high diagnostic rates, early access to novel therapies, and widespread awareness of CTCL
- The U.S. holds a significant share of 79.3%, driven by payer support, specialist density, and FDA-led drug acceleration pathways for rare hematologic malignancies
- Presence of key innovators such as Kyowa Kirin, Soligenix, and Seattle Genetics bolsters the competitive landscape and supports consistent therapeutic innovation
- The rising number of dermatologic oncology centers and focus on personalized cancer care further reinforce North America’s leadership position
“Asia-Pacific is Projected to Register the Highest CAGR in the Sézary Syndrome Market”
- Asia-Pacific is expected to witness the highest growth rate due to increased CTCL diagnosis, regional cancer network expansion, and growing availability of international treatment guidelines
- Countries like Japan, South Korea, and India are strengthening oncology reimbursement frameworks and investing in molecular diagnostic platforms
- Japan, with its robust biopharma sector and aging population, remains a major driver for CTCL therapy adoption and drug development
- With expanding access to oncology services, the APAC region is positioned as the fastest-growing market in Sézary Syndrome treatment over the forecast period
Sézary Syndrome Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Kyowa Kirin Co., Ltd. (Japan)
- Soligenix, Inc. (U.S.)
- Miragen Therapeutics, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Spectrum Pharmaceuticals, Inc. (U.S.)
- Helsinn Healthcare SA (Switzerland)
- Innate Pharma SA (France)
- Seagen Inc. (U.S.)
- Gilead Sciences, Inc. (U.S.)
- Novartis AG (Switzerland)
- Johnson & Johnson Services, Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Pfizer Inc. (U.S.)
- Eisai Co., Ltd. (Japan)
- Hikma Pharmaceuticals PLC (U.K.)
- Bayer AG (Germany)
- Shionogi Inc. (Japan)
- Amerigen Pharmaceuticals Limited (U.S.)
- STI Pharma, LLC (U.S.)
- Minophagen Pharmaceutical Co., Ltd. (Japan)
- Bioniz Therapeutics (U.S.)
- BE Biopharma (U.S.)
- 4SC AG (Germany)
- Asher Biotherapeutics, Inc. (U.S.)
Latest Developments in Global Sézary Syndrome Market
- In February 2025, Kyowa Kirin announced Phase III results for its investigational antibody therapy in Sézary Syndrome, demonstrating improved overall response rate and durable symptom relief. This advancement strengthens clinical confidence and is expected to accelerate regulatory approvals, boosting market uptake
- In November 2024, Soligenix received Fast Track Designation from the U.S. FDA for SGX301, a synthetic hypericin-based photodynamic therapy for treatment-resistant CTCL. This designation speeds up the development timeline, potentially bringing innovative therapies to market faster and expanding treatment options
- In September 2024, Helsinn and a U.S.-based academic center launched a joint clinical trial investigating an oral HDAC inhibitor combination regimen for Sézary Syndrome. This collaboration is expected to drive innovation in oral treatment regimens, improving patient convenience and expanding the addressable market
- In June 2024, Innate Pharma received orphan drug designation for its anti-KIR3DL2 antibody IPH4102, advancing its CTCL pipeline in both Europe and the U.S. The designation provides development incentives, enhancing the commercial viability and market penetration of novel CTCL therapies
- In April 2024, Seattle Genetics expanded its licensing agreement with Takeda to include Asia-Pacific development rights for brentuximab vedotin in CTCL indications, including Sézary Syndrome. This strategic expansion broadens geographic access, supporting regional market growth and increased drug availability in high-need areas
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.