Global Tasigna Market
Market Size in USD Billion
CAGR :
% 
 USD
2.17 Billion
USD
3.72 Billion
2025
2033
USD
2.17 Billion
USD
3.72 Billion
2025
2033
| 2026 –2033 | |
| USD 2.17 Billion | |
| USD 3.72 Billion | |
|
|
|
|
Tasigna Market Size
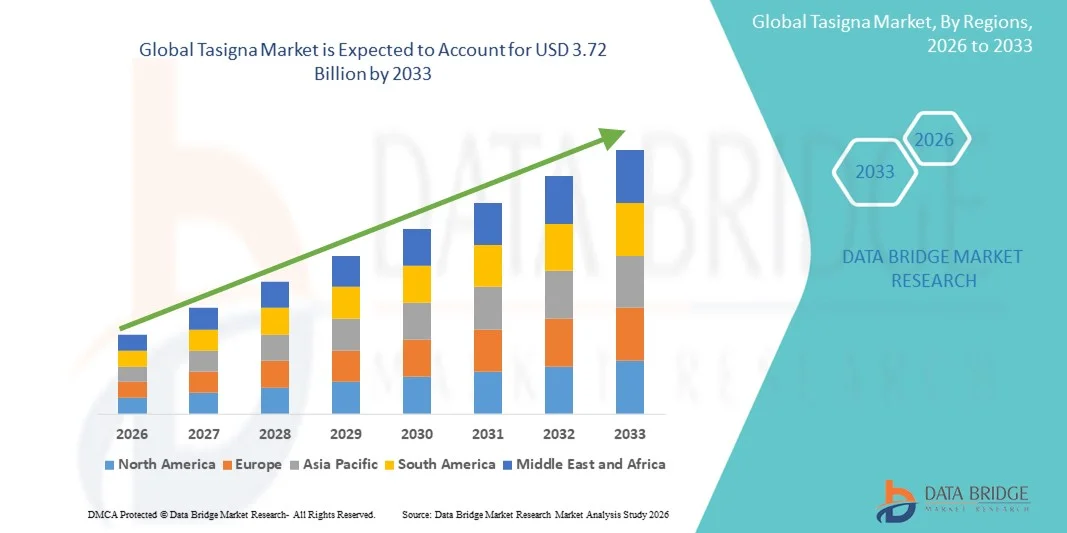
- The global Tasigna market size was valued at USD 2.17 billion in 2025 and is expected to reach USD 3.72 billion by 2033, at a CAGR of 7.0% during the forecast period
- The market growth is largely fueled by the rising prevalence of chronic myeloid leukemia (CML) worldwide and the increasing adoption of targeted therapies that offer improved efficacy and reduced adverse effects compared to conventional treatments
- Furthermore, advancements in oncology research, expanding access to cancer therapeutics, and growing physician preference for second-generation tyrosine kinase inhibitors (TKIs) are strengthening Tasigna’s clinical uptake. These converging factors are accelerating the demand for Tasigna therapy, thereby significantly boosting the industry’s overall growth
Tasigna Market Analysis
- Tasigna (nilotinib), a second-generation tyrosine kinase inhibitor, has become a critical therapy in the management of Philadelphia chromosome–positive chronic myeloid leukemia (CML), widely used across global oncology practices due to its higher potency, improved molecular response rates, and suitability for treatment-free remission programs compared to earlier TKIs
- The growing burden of leukemia, increased adoption of targeted therapies, and expanding clinical use of TKIs as first-line and second-line treatments are key factors accelerating Tasigna uptake, supported by favorable treatment guidelines and improved patient awareness regarding precision oncology
- North America dominated the Tasigna market with the largest revenue share of 41.8% in 2025, driven by high diagnosis rates, strong healthcare infrastructure, early adoption of novel oncology therapeutics, and consistent regulatory support, with the U.S. showing substantial demand as physicians increasingly prefer TKIs with proven long-term molecular remission benefits and established safety profiles
- Asia-Pacific is expected to be the fastest growing region during the forecast period due to rising cancer incidence, expanding access to specialty oncology drugs, rapid improvements in healthcare spending, and broader availability of branded and reimbursed targeted therapies for CML
- The Chronic Myeloid Leukemia (CML) segment dominated the Tasigna market with a market share of 58.7% in 2025, supported by strong clinical evidence demonstrating Tasigna’s effectiveness in achieving deep and durable molecular responses, its role in treatment-free remission strategies, and its widespread adoption as a preferred therapy in newly diagnosed CML patients
Report Scope and Tasigna Market Segmentation
|
Attributes |
Tasigna Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Tasigna Market Trends
Increasing Shift Toward Treatment-Free Remission and Precision Oncology Integration
- A significant and accelerating trend in the global Tasigna market is the growing adoption of treatment-free remission (TFR) strategies and deeper alignment with precision oncology protocols, allowing eligible chronic myeloid leukemia (CML) patients to discontinue therapy under controlled molecular monitoring
- For instance, Tasigna’s inclusion in TFR pathways endorsed by leading oncology associations has strengthened its clinical relevance, enabling patients who achieve sustained deep molecular responses to reduce long-term treatment burden
- Precision oncology integration enables features such as molecular response tracking, optimized dose management, and improved patient stratification, with Tasigna demonstrating strong outcomes in achieving rapid and durable molecular responses compared to earlier TKIs
- The seamless integration of Tasigna into multidisciplinary cancer care spanning molecular diagnostics, digital monitoring tools, and personalized treatment algorithms is creating more connected, data-driven CML management pathways
- This trend toward more targeted, remission-oriented, and digitally supported leukemia treatment frameworks is reshaping patient expectations, prompting drug developers to enhance TKI-response analytics and long-term remission support programs
- Growing patient engagement through digital health apps and remote monitoring platforms is driving better adherence and real-world outcome tracking, further reinforcing Tasigna’s role in precision leukemia care
- The expansion of tele-oncology services in emerging markets is facilitating wider access to Tasigna, enabling physicians to monitor molecular responses remotely and guide therapy adjustments efficiently
Tasigna Market Dynamics
Driver
Growing Demand Driven by Rising CML Diagnosis Rates and Expansion of Targeted Therapy Adoption
- The increasing prevalence of CML globally, coupled with expanding adoption of targeted therapies such as second-generation TKIs, is a significant driver accelerating demand for Tasigna in both first-line and second-line treatment settings
- For instance, in recent years, several oncology centers have expanded access to molecular testing platforms, enabling earlier CML detection and improving patient eligibility for advanced treatments such as Tasigna
- As healthcare systems introduce more efficient diagnostic workflows and clinicians emphasize precision-guided treatment, Tasigna offers established benefits such as rapid cytogenetic responses and stronger long-term remission outcomes
- Furthermore, rising awareness of treatment-free remission programs and strong guideline support for Tasigna in chronic-phase CML are positioning the therapy as a preferred choice among oncologists worldwide
- The convenience of oral administration, predictable safety profile, and robust post-marketing evidence supporting its clinical efficacy are key factors propelling Tasigna’s increasing adoption across diverse healthcare markets
- Growing investments in oncology education and patient support programs are enhancing awareness about Tasigna’s benefits, improving therapy initiation and long-term adherence rates
- Expanding collaborations between pharmaceutical companies and cancer care networks are facilitating broader Tasigna distribution and access, particularly in underserved regions with rising CML incidence
Restraint/Challenge
Cardiovascular Safety Concerns and Regulatory Oversight Requirements
- Concerns surrounding cardiovascular-related adverse effects associated with Tasigna therapy pose a significant challenge, necessitating careful patient selection and regular monitoring to ensure treatment safety
- For instance, published clinical data highlighting risks such as arterial occlusive events have raised caution among clinicians when prescribing Tasigna to patients with pre-existing cardiovascular conditions
- Addressing these safety concerns through enhanced patient education, risk-mitigation protocols, and regular molecular and cardiovascular assessments is critical for maintaining clinician and patient confidence
- In addition, the stringent regulatory requirements governing oncology drug safety, including mandated long-term follow-up and risk-management documentation, may slow therapy expansion in some regions
- While treatment alternatives exist, the perceived cardiovascular risk associated with Tasigna can influence prescribing behaviors, particularly in populations requiring long-term TKI therapy
- Overcoming these challenges through improved real-world evidence, updated safety guidelines, and ongoing innovation in TKI formulations will be vital for sustaining Tasigna’s growth trajectory in global oncology markets
- The high long-term treatment costs associated with Tasigna, especially in regions with limited reimbursement frameworks, continue to restrict access for many patients and place financial pressure on healthcare systems.
- Competition from alternative TKIs including generics and newer agents with differentiated safety profiles poses a growing challenge, as clinicians may opt for therapies with lower cardiovascular risk or more flexible dosing options depending on patient characteristics
Tasigna Market Scope
The market is segmented on the basis of type, application, dosage, route of administration, demographic, side effects, end-users, and distribution channel.
- By Type
On the basis of type, the Tasigna market is segmented into 50mg, 200mg, and others. The 200mg segment dominated the market with the largest market revenue share of 62.5% in 2025, driven by its established efficacy as the standard dosage for adult chronic-phase CML patients. Oncologists often prioritize 200mg Tasigna for achieving rapid and deep molecular responses, as clinical guidelines recommend it for first-line and certain second-line treatments. The dominance of this dosage is also supported by its widespread availability, robust clinical evidence, and strong patient adherence due to fewer required adjustments. In addition, patient familiarity and historical prescribing patterns reinforce its leading position. Health systems and oncology centers prefer 200mg formulations for bulk procurement and standardized treatment protocols.
The 50mg segment is expected to witness the fastest growth rate of 19.8% from 2026 to 2033, fueled by increasing adoption in dose-adjusted therapy for pediatric and elderly patients with comorbidities. Lower-dose Tasigna allows safer titration and better tolerance in patients at risk of cardiovascular events or other side effects. Growing awareness among clinicians of personalized dosing strategies supports uptake in specialized populations. Regulatory approvals for flexible dosing in emerging markets are further contributing to this segment’s rapid expansion. The segment also benefits from initiatives targeting safer long-term therapy and improved quality-of-life management for sensitive patient groups.
- By Application
On the basis of application, the Tasigna market is segmented into Chronic Myeloid Leukemia (CML) and others. The Chronic Myeloid Leukemia (CML) segment dominated the market with a share of 58.7% in 2025, as Tasigna is specifically approved for Philadelphia chromosome–positive CML treatment. Its proven ability to induce deep molecular responses, achieve long-term remission, and support treatment-free remission strategies drives dominance. The segment also benefits from high disease prevalence, standardized treatment guidelines, and strong physician preference for second-generation TKIs. Patient adherence and clinician confidence further reinforce its leading market position. In addition, clinical data demonstrating superior efficacy over first-generation TKIs strengthens Tasigna’s dominance.
The others segment is expected to witness the fastest growth rate of 15.4% from 2026 to 2033, driven by increasing off-label exploration and clinical trials targeting other hematologic malignancies. Research into combination therapy and expanded indications for rare leukemias is creating new revenue streams. Greater awareness of Tasigna’s mechanism of action encourages trial usage in related conditions. Emerging markets are gradually adopting these applications as regulatory approvals broaden. The potential for therapy diversification contributes to segment growth alongside increasing investment in oncology R&D.
- By Dosage
On the basis of dosage, the Tasigna market is segmented into tablet, injection, and others. The tablet segment dominated the market with a share of 94.2% in 2025, as oral administration is the standard route for Tasigna, offering convenience and improved patient adherence compared to invasive forms. Tablets allow flexible dosing schedules and facilitate home-based treatment, which reduces hospital visits. The dominance is further reinforced by long-term clinical data supporting safety and efficacy. Tablets are widely preferred by both clinicians and patients for chronic management. Standardized oral formulations simplify procurement, storage, and distribution in healthcare systems.
The injection segment is expected to witness the fastest CAGR of 12.6% from 2026 to 2033, primarily driven by research into injectable formulations for rapid systemic delivery in acute scenarios. Injectable forms are also being explored for patients with absorption issues or gastrointestinal complications. Ongoing clinical studies targeting niche patient populations further boost interest. Improved bioavailability and targeted delivery methods increase their potential adoption. Healthcare providers in specialized oncology centers are key drivers of this emerging segment.
- By Route of Administration
On the basis of route of administration, the Tasigna market is segmented into oral, intravenous, and others. The oral segment dominated the market with a share of 93% in 2025, as Tasigna’s standard prescription is orally administered, allowing convenient home-based therapy for chronic-phase CML patients. Oral administration ensures continuous therapy adherence, reduced hospital dependence, and easier dose adjustments. The segment’s dominance is also supported by high physician confidence and patient preference. Regulatory guidelines and clinical trials predominantly focus on oral usage. Pharmaceutical companies emphasize oral formulations in marketing and distribution strategies.
The intravenous segment is expected to witness the fastest CAGR of 11.9% from 2026 to 2033, driven by research into novel delivery mechanisms for acute or high-risk leukemia cases. IV administration provides rapid bioavailability and is being explored for combination therapies. Hospitals and specialty oncology centers are early adopters of IV formulations. Clinical trials in emerging markets are expected to support future growth. The segment is further supported by interest in patient populations who require precise dosing adjustments under supervision.
- By Demographic
On the basis of demographic, the Tasigna market is segmented into adults and pediatric. The adult segment dominated the market with a share of 88.4% in 2025, driven by the high prevalence of CML among adults and the approval of standard Tasigna doses for this population. Adults demonstrate higher adherence rates to oral TKIs, supported by monitoring and long-term follow-up. Physician prescribing patterns favor adults due to robust clinical evidence and guideline support. The segment also benefits from insurance coverage and established treatment protocols. In addition, adults form the core patient base targeted by awareness and patient support programs.
The pediatric segment is expected to witness the fastest CAGR of 16.2% from 2026 to 2033, fueled by increasing pediatric CML diagnosis and tailored dosing approvals. Clinicians are adopting lower-dose regimens (e.g., 50mg) to reduce side effects in children. Pediatric oncology centers are expanding access to Tasigna under clinical supervision. Growing awareness among caregivers and regulatory approvals in emerging markets support rapid uptake. Pediatric-focused patient support initiatives are further encouraging adoption.
- By Side Effects
On the basis of side effects, the Tasigna market is segmented into headache, nausea, vomiting, constipation, cough, diarrhea, rash, night sweats, muscle pain, tiredness, temporary hair loss, sore throat, runny/stuffy nose, sneezing, and others. The nausea segment dominated the market with a share of 34.5% in 2025, as it is the most commonly reported side effect influencing adherence and patient management strategies. Clinicians monitor this closely to optimize dosing and provide supportive care. Pharmaceutical companies include antiemetic guidance in labeling and patient education. Awareness of nausea management improves therapy continuity. The dominance is further reinforced by robust reporting and real-world evidence tracking.
The rash segment is expected to witness the fastest CAGR of 13.7% from 2026 to 2033, driven by growing recognition of dermatologic reactions as a key adverse event and increasing emphasis on monitoring and early intervention. Studies are exploring strategies to mitigate rash without discontinuing therapy. Patient awareness campaigns help manage expectations. Dermatology consultations integrated with oncology care are becoming more common. Emerging markets are seeing improved reporting and management, supporting growth.
- By End-Users
On the basis of end-users, the Tasigna market is segmented into clinic, hospital, and others. The hospital segment dominated the market with a share of 79.6% in 2025, as most CML patients require diagnosis, regular molecular monitoring, and long-term prescription oversight. Hospitals provide comprehensive care including counseling, laboratory tests, and adherence monitoring. The segment’s dominance is reinforced by insurance reimbursement schemes and integrated oncology services. Hospitals are primary distributors of Tasigna in both urban and semi-urban regions. Specialist hematology/oncology centers prefer hospital-based administration.
The clinic segment is expected to witness the fastest CAGR of 14.5% from 2026 to 2033, driven by the expansion of outpatient oncology and hematology clinics capable of dispensing Tasigna. Clinics enable closer patient follow-up, convenient prescription refills, and supportive care management. Telemedicine integration in clinics enhances adherence and monitoring. Growing awareness of CML among local clinics contributes to rapid adoption. Clinics in emerging markets are increasingly serving as key access points for Tasigna therapy.
- By Distribution Channel
On the basis of distribution channel, the Tasigna market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market with a share of 81.2% in 2025, as hospitals remain the primary point of distribution for specialty oncology drugs such as Tasigna, ensuring patient adherence and proper monitoring. Hospital pharmacies provide regulated access, guidance on side-effect management, and stock for long-term therapy. Bulk procurement and standardized dosing in hospitals reinforce dominance. Hospital-based dispensing also integrates patient support programs. This channel ensures secure supply and adherence tracking.
The online pharmacy segment is expected to witness the fastest CAGR of 17.3% from 2026 to 2033, driven by growing e-pharmacy penetration, telemedicine services, and increasing patient preference for home delivery of chronic medications. Online platforms provide convenience, confidentiality, and easier access in remote areas. Regulatory approvals for secure online prescription fulfillment are supporting growth. Integration with digital health records enhances patient safety and adherence. Expansion of online healthcare infrastructure in emerging markets is further accelerating adoption.
Tasigna Market Regional Analysis
- North America dominated the Tasigna market with the largest revenue share of 41.8% in 2025, driven by high diagnosis rates, strong healthcare infrastructure, early adoption of novel oncology therapeutics, and consistent regulatory support
- Patients and clinicians in the region benefit from widespread availability of molecular diagnostic tools, well-established treatment protocols, and access to specialized oncology centers, which support effective monitoring and long-term therapy with Tasigna
- This dominance is further reinforced by strong insurance coverage, government support for cancer care, and growing awareness of treatment-free remission programs, positioning Tasigna as a preferred therapy for both newly diagnosed and resistant CML patients
U.S. Tasigna Market Insight
The U.S. Tasigna market captured the largest revenue share of 83% in 2025 within North America, fueled by the high prevalence of chronic myeloid leukemia (CML) and advanced healthcare infrastructure. Patients increasingly prioritize targeted therapy with second-generation TKIs due to their efficacy in achieving deep molecular responses. The growing focus on treatment-free remission programs, combined with widespread availability of molecular diagnostic tools and specialized oncology centers, further propels Tasigna adoption. Moreover, strong insurance coverage, government initiatives supporting cancer care, and physician preference for evidence-based therapies are significantly contributing to the market's expansion.
Europe Tasigna Market Insight
The Europe Tasigna market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing CML diagnosis rates and growing adoption of targeted therapies. Rising awareness among patients and clinicians about treatment-free remission programs is fostering the uptake of Tasigna. European healthcare systems emphasize precision oncology, supporting early diagnosis and optimized treatment protocols. The region is experiencing significant growth across hospital and clinic settings, with Tasigna increasingly integrated into first-line and second-line CML treatment plans. In addition, government support for oncology drug reimbursement and guideline-driven prescribing are expected to sustain market growth.
U.K. Tasigna Market Insight
The U.K. Tasigna market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising CML prevalence and the expanding use of second-generation TKIs. Concerns regarding disease progression and resistance to first-generation therapies are encouraging clinicians to adopt Tasigna for both newly diagnosed and resistant patients. The U.K.’s robust healthcare infrastructure, along with strong patient awareness and access to specialty oncology centers, is expected to continue to stimulate market growth. In addition, the integration of molecular monitoring and treatment-free remission protocols strengthens the adoption of Tasigna in clinical practice.
Germany Tasigna Market Insight
The Germany Tasigna market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of advanced CML treatments and the demand for precision-targeted therapies. Germany’s well-developed healthcare infrastructure, coupled with a strong emphasis on research and innovation in oncology, promotes Tasigna adoption, particularly in hospital and specialized clinic settings. The integration of molecular diagnostics and treatment monitoring is becoming increasingly prevalent, supporting clinicians in optimizing dosing strategies. Growing patient education programs and guideline-driven therapy further align with local clinical expectations.
Asia-Pacific Tasigna Market Insight
The Asia-Pacific Tasigna market is poised to grow at the fastest CAGR of 21.5% from 2026 to 2033, driven by increasing CML incidence, expanding healthcare infrastructure, and rising awareness of targeted therapies in countries such as China, Japan, and India. The region's growing access to molecular diagnostics and oncology specialists is driving Tasigna adoption. Furthermore, government initiatives promoting cancer care, improved reimbursement programs, and partnerships with international pharmaceutical companies are expanding patient access. Emerging markets are also witnessing growth due to rising affordability and availability of branded and generic Tasigna formulations.
Japan Tasigna Market Insight
The Japan Tasigna market is gaining momentum due to the country’s advanced healthcare system, high awareness of CML management, and increasing adoption of second-generation TKIs. Patients and clinicians prioritize targeted therapies for achieving sustained molecular responses and supporting treatment-free remission programs. The integration of molecular monitoring and personalized dosing strategies is fueling growth. Moreover, the aging population in Japan is such asly to drive demand for safe, effective, and easy-to-administer oral therapies in both hospital and outpatient settings. Expanding oncology centers and patient support programs further bolster market growth.
India Tasigna Market Insight
The India Tasigna market accounted for the largest market revenue share in Asia Pacific in 2025, attributed to rising CML prevalence, improving healthcare infrastructure, and growing awareness of targeted therapy benefits. India is witnessing an increase in patients seeking advanced oncology treatments, supported by expanding access to molecular diagnostics and specialized cancer centers. The push towards affordable cancer care, combined with domestic availability of branded and generic Tasigna formulations, is propelling market adoption. In addition, government healthcare initiatives and rising patient awareness programs are key factors driving Tasigna uptake across hospital and clinic settings.
Tasigna Market Share
The Tasigna industry is primarily led by well-established companies, including:
- Novartis AG (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- Pfizer Inc. (U.S.)
- AstraZeneca (U.K.)
- Merck & Co., Inc. (U.S.)
- Amgen Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Gilead Sciences, Inc. (U.S.)
- Sanofi (France)
- Eli Lilly and Company (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Teva Pharmaceutical Industries Ltd. (Israel)
- AbbVie Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Bayer AG (Germany)
- GSK plc (U.K.)
- Biogen Inc. (U.S.)
- Daiichi Sankyo Company, Limited (Japan)
- Hospira, Inc. (U.S.)
What are the Recent Developments in Global Tasigna Market?
- In October 2025, the U.S. Food and Drug Administration (FDA) accepted a new drug application (NDA) for an alternative formulation of nilotinib (code‑named XS003) developed by Xspray Pharma, intended for treatment of Chronic Myeloid Leukemia (CML)
- In May 2025, Apotex Corp. launched the first generic version of Tasigna (nilotinib) in the United States with 180 days of market exclusivity. This is important because it introduces a more affordable form of a critical leukemia therapy, which may improve access for both adult and pediatric chronic myeloid leukemia (CML) patients
- In December 2024, the ATOM Coalition welcomed the FDA’s approval of generic nilotinib, highlighting it as a breakthrough in expanding access to affordable cancer treatment worldwide. The approval was positively viewed as a public‑health win, especially for low‑ and middle‑income countries where access to expensive branded therapies has been limited
- In November 2024, Azurity Pharmaceuticals, Inc. received approval from U.S. Food and Drug Administration (FDA) for a reformulated nilotinib product Danziten which does not require the stringent fasting (mealtime) restrictions associated with Tasigna. This regulatory approval is a patient‑convenience milestone, as it may improve adherence and quality of life for CML patients
- In June 2023, Medicines Patent Pool (MPP) signed sublicence agreements with multiple manufacturers. These are the first sublicence agreements for a cancer drug under MPP a move expected to substantially broaden global access to Tasigna therapy in underserved regions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.