Global Transcatheter Edge To Edge Repair Teer Devices Market
Market Size in USD Million
CAGR :
% 
 USD
990.00 Million
USD
3,313.39 Million
2024
2032
USD
990.00 Million
USD
3,313.39 Million
2024
2032
| 2025 –2032 | |
| USD 990.00 Million | |
| USD 3,313.39 Million | |
|
|
|
|
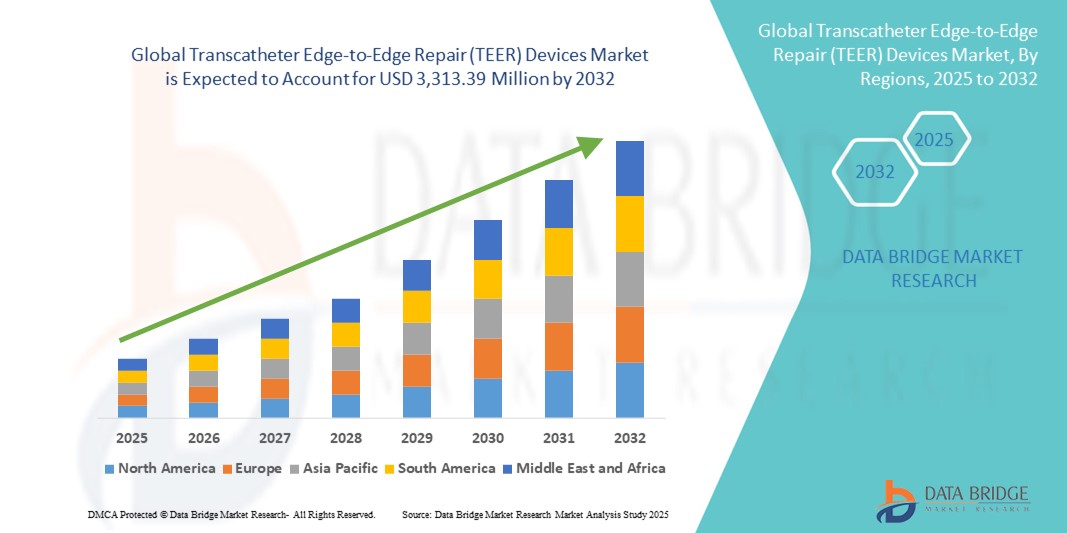
Transcatheter Edge-to-Edge Repair (TEER) Devices Market Size
- The global Transcatheter Edge-to-Edge Repair (TEER) devices market size was valued at USD 990 million in 2024 and is expected to reach USD 3,313.39 million by 2032, at a CAGR of 16.30% during the forecast period
- The market growth is largely driven by the rising prevalence of mitral and tricuspid regurgitation, coupled with the increasing adoption of minimally invasive cardiac interventions that reduce hospital stays and recovery time
- Furthermore, continuous advancements in device design, imaging guidance, and procedural techniques alongside growing geriatric populations and broader regulatory approvals are positioning TEER devices as a preferred treatment option for high-risk patients. These combined factors are accelerating procedural volumes and significantly boosting the industry’s expansion
Transcatheter Edge-to-Edge Repair (TEER) Devices Market Analysis
- Transcatheter edge-to-edge repair (TEER) devices, offering percutaneous, minimally invasive repair for mitral and tricuspid valve regurgitation, are increasingly vital components of modern structural-heart care in both hospitals and specialized cardiac centres due to shorter recovery times, lower procedural risk for high-risk patients, and improved quality-of-life outcomes.
- The accelerating demand for TEER devices is primarily fueled by the rising prevalence of valvular heart diseases, an ageing population, broader clinical indications, and the clinical shift toward less invasive structural-heart interventions
- North America dominated the Transcatheter Edge-to-Edge Repair (TEER) devices market with the largest revenue share of 30.8% in 2024, supported by advanced healthcare infrastructure, early adoption of novel cardiovascular technologies, favorable reimbursement dynamics, and a strong presence of leading device manufacturers, with the U.S. experiencing significant growth in procedural volumes driven by technology upgrades and expanding clinical indications
- Asia-Pacific is expected to be the fastest growing region in the Transcatheter Edge-to-Edge Repair (TEER) devices market during the forecast period due to improving access to interventional cardiology, rising healthcare expenditures, growing awareness of minimally invasive valve therapies, and expanding procedural capacity
- The mitral regurgitation segment dominated the transcatheter edge-to-edge repair (TEER) devices market with a market share of 70.5% in 2024, driven by its high prevalence and strong clinical validation of TEER as a safe and effective treatment for patients unsuitable for open-heart surgery
Report Scope and Transcatheter Edge-to-Edge Repair (TEER) Devices Market Segmentation
|
Attributes |
Transcatheter Edge-to-Edge Repair (TEER) Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Transcatheter Edge-to-Edge Repair (TEER) Devices Market Trends
Technological Advancements Enhancing Procedural Precision and Patient Outcomes
- A significant and accelerating trend in the global TEER devices market is the integration of advanced imaging modalities, catheter navigation systems, and next-generation clip designs, which are improving procedural accuracy and expanding the range of treatable valve anatomies. This technological progression is enhancing procedural success rates and long-term outcomes for patients with mitral and tricuspid regurgitation
- For instance, Abbott’s latest generation MitraClip G4 system offers independent leaflet grasping and expanded size options, enabling greater customization for complex anatomies. Similarly, Edwards Lifesciences is developing Pascal Precision with enhanced steering and control features, facilitating optimal device placement
- Integration of real-time 3D transesophageal echocardiography (TEE) and advanced fluoroscopy allows interventional cardiologists to visualize device positioning with high precision, reducing procedure times and improving patient safety. Furthermore, device design improvements are enabling treatment of anatomies previously unsuitable for TEER, such as complex tricuspid regurgitation cases
- The convergence of device innovation with procedural imaging integration is also fostering the use of TEER in hybrid cardiac procedures and broadening adoption beyond top-tier cardiac centers into more community-based facilities
- This trend toward more precise, adaptable, and user-friendly TEER systems is fundamentally reshaping physician capabilities and patient eligibility criteria. Consequently, companies such as Abbott, Edwards Lifesciences, and JenaValve are investing heavily in R&D to introduce devices with improved durability, greater leaflet capture ranges, and compatibility with expanded indications
- The demand for TEER systems with enhanced procedural control and imaging integration is growing rapidly across both mature and emerging cardiac care markets, as hospitals and healthcare systems aim to improve patient outcomes while minimizing procedural risk
Transcatheter Edge-to-Edge Repair (TEER) Devices Market Dynamics
Driver
Rising Prevalence of Valvular Heart Disease and Shift Toward Minimally Invasive Interventions
- The increasing prevalence of mitral and tricuspid regurgitation, especially among the ageing global population, is a significant driver for the growing demand for TEER devices
- For instance, epidemiological studies suggest that over 10% of individuals above 75 years suffer from clinically significant mitral regurgitation, creating a substantial treatment pool for TEER. The minimally invasive nature of these procedures offers an attractive alternative to open-heart surgery, particularly for high-risk patients with comorbidities
- As healthcare systems prioritize less invasive interventions to reduce hospital stays and recovery times, TEER has emerged as a key therapeutic option supported by positive long-term clinical data
- Furthermore, increasing physician training, supportive reimbursement policies in developed regions, and expanding clinical indications for both mitral and tricuspid repair are further boosting adoption
- The ability to offer percutaneous treatment to patients previously deemed untreatable with surgery is accelerating procedural volumes and reinforcing TEER’s role in the structural heart portfolio
Restraint/Challenge
High Device Costs and Limited Accessibility in Emerging Markets
- The relatively high cost of TEER devices and procedures poses a significant barrier to widespread adoption, particularly in low- and middle-income countries. In many emerging markets, limited healthcare budgets, scarcity of specialized cardiac centers, and lack of trained interventional cardiologists restrict access to these advanced therapies
- For instance, while North America and Western Europe benefit from established reimbursement frameworks, many Asia-Pacific, Latin American, and African healthcare systems face financial constraints that hinder large-scale TEER deployment
- In addition, the learning curve associated with TEER procedures demands specialized training and experience, limiting adoption to high-volume, well-equipped cardiac centers
- Addressing these challenges through cost optimization, technology transfer partnerships, physician training programs, and government-backed funding initiatives will be vital to expanding the global TEER footprint
- Without these measures, disparities in access may persist, slowing market penetration in high-growth but resource-constrained regions
Transcatheter Edge-to-Edge Repair (TEER) Devices Market Scope
The market is segmented on the basis of device type, application, procedure type, and end user.
- By Device Type
On the basis of device type, the Transcatheter Edge-to-Edge Repair (TEER) devices market is segmented into clip-based systems and other innovative devices. The clip-based systems segment dominated the market with the largest market revenue share in 2024, driven by the widespread clinical adoption of devices such as MitraClip and Pascal for the treatment of mitral regurgitation. Their strong safety profile, high procedural success rates, and robust clinical evidence base have established them as the gold standard for TEER procedures. Growing physician familiarity and the availability of advanced generations with improved leaflet grasping and delivery precision further reinforce the dominance of this segment.
The other innovative devices segment is anticipated to witness the fastest growth from 2025 to 2032, fueled by ongoing R&D in alternative leaflet approximation technologies and novel repair concepts. Devices targeting complex anatomies, tricuspid regurgitation, and patients with prior interventions are gaining traction, supported by increasing clinical trials and expanding regulatory approvals.
- By Application
On the basis of application, the Transcatheter Edge-to-Edge Repair (TEER) devices market is segmented into mitral regurgitation and tricuspid regurgitation. The mitral regurgitation segment accounted for the largest market revenue share of 70.5% in 2024 due to the high prevalence of the disease and the extensive clinical validation of TEER as a first-line treatment for high-risk surgical candidates. The growing pool of elderly patients with degenerative and functional MR, coupled with guideline endorsements, continues to drive demand.
The tricuspid regurgitation segment is expected to register the fastest growth during the forecast period, supported by the emergence of dedicated tricuspid TEER devices, increasing awareness of the clinical burden of tricuspid disease, and early positive outcomes from pivotal trials.
- By Procedure Type
On the basis of procedure type, the Transcatheter Edge-to-Edge Repair (TEER) devices market is segmented into transfemoral TEER and transapical TEER. The transfemoral TEER segment dominated the market in 2024, favored for its less invasive nature, shorter recovery times, and reduced procedural risk compared to transapical approaches. The transfemoral route’s compatibility with advanced catheter-based delivery systems and its suitability for a wide range of anatomies have made it the preferred choice in most centers.
The transapical TEER segment is projected to grow steadily during forecast period, primarily serving patients with challenging anatomies or cases where transfemoral access is not feasible. Technological advancements aimed at reducing procedural invasiveness and improving patient safety may further support its adoption in niche clinical settings.
- By End User
On the basis of end user, the Transcatheter Edge-to-Edge Repair (TEER) devices market is segmented into hospitals, ambulatory surgical centers, cardiac catheterization labs, cardiology clinics, and research institutions. The hospitals segment held the largest market share in 2024, supported by their access to advanced imaging technologies, multidisciplinary cardiac teams, and the infrastructure needed for complex TEER procedures. Reimbursement availability and higher patient inflow for structural heart interventions further contribute to their dominance.
The cardiac catheterization labs segment is expected to record notable growth through 2032, driven by the shift toward catheter-based therapies, increased investment in interventional facilities, and training programs expanding TEER procedural capabilities beyond traditional operating rooms.
Transcatheter Edge-to-Edge Repair (TEER) Devices Market Regional Analysis
- North America dominated the Transcatheter Edge-to-Edge Repair (TEER) devices market with the largest revenue share of 30.8% in 2024, supported by advanced healthcare infrastructure, early adoption of novel cardiovascular technologies, favorable reimbursement dynamics, and a strong presence of leading device manufacturers
- Healthcare providers in the region benefit from advanced cardiac care infrastructure, favorable reimbursement frameworks, and a growing pool of skilled interventional cardiologists, enabling faster adoption of TEER technology in both mitral and tricuspid valve repair cases
- This leadership position is further reinforced by strong clinical research activities, early regulatory approvals, and increasing patient awareness regarding less invasive treatment alternatives, solidifying North America as the primary hub for TEER device innovation and deployment across diverse healthcare settings
U.S. Transcatheter Edge-to-Edge Repair (TEER) Devices Market Insight
The U.S. Transcatheter Edge-to-Edge Repair (TEER) devices market captured the largest revenue share of 27.8% in 2024 within North America, fueled by rapid adoption of minimally invasive structural-heart therapies and extensive procedural capacity at tertiary cardiac centers. Providers and patients increasingly prefer TEER for high-risk mitral and tricuspid regurgitation cases due to shorter hospital stays and favorable clinical outcomes. The strong ecosystem of clinical training, active investigator-led trials, and established reimbursement pathways further propels procedural volumes and technology uptake. Moreover, continued device innovation and physician experience are significantly contributing to market expansion.
Europe Transcatheter Edge-to-Edge Repair (TEER) Devices Market Insight
The Europe Transcatheter Edge-to-Edge Repair (TEER) devices market is projected to expand at a substantial CAGR throughout the forecast period, driven by coordinated healthcare networks, centralized regulatory pathways and growing investments in structural-heart programs. Increasing establishment of specialized TEER centers and “clip labs” across major healthcare systems is enabling broader clinical access. European payor systems and clinician guidelines that support minimally invasive alternatives are fostering uptake across both public and private hospitals.
U.K. Transcatheter Edge-to-Edge Repair (TEER) Devices Market Insight
The U.K. Transcatheter Edge-to-Edge Repair (TEER) devices market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by the NHS emphasis on minimally invasive care pathways and regional centers of excellence for structural heart disease. Rising awareness of percutaneous options among cardiologists and referral networks, together with expanding training programs, are encouraging greater procedural adoption. In addition, centralized procurement and evidence-driven commissioning are expected to streamline access in both tertiary and regional centers.
Germany Transcatheter Edge-to-Edge Repair (TEER) Devices Market Insight
The Germany Transcatheter Edge-to-Edge Repair (TEER) devices market is expected to expand at a considerable CAGR during the forecast period, driven by a strong cardiac care infrastructure, high procedure volumes, and early adoption of innovative devices. Germany’s network of university hospitals and specialized cardiology clinics supports rapid clinical translation and multi-center studies, promoting clinician confidence in TEER. Integration of advanced imaging and hybrid interventional suites further enhances procedural success and scalability.
Asia-Pacific Transcatheter Edge-to-Edge Repair (TEER) Devices Market Insight
The Asia-Pacific Transcatheter Edge-to-Edge Repair (TEER) devices market is poised to grow at the fastest CAGR of 12% during the forecast period, driven by rising prevalence of valvular disease, expanding catheterization lab capacity, and growing healthcare expenditure across China, Japan, India, and Southeast Asia. Government initiatives to upgrade tertiary care, increasing training of interventional cardiologists, and expanding clinical trial activity are accelerating adoption. As regional centers build expertise, TEER is transitioning from niche to more mainstream structural-heart care.
Japan Transcatheter Edge-to-Edge Repair (TEER) Devices Market Insight
The Japan Transcatheter Edge-to-Edge Repair (TEER) devices market is gaining momentum due to advanced cardiovascular care, an ageing population with higher valvular disease burden, and strong clinical research activity. Japanese hospitals emphasize precision imaging and multidisciplinary heart teams, which aligns well with TEER procedural requirements. The result is steadily increasing procedural volumes and broader adoption across major cardiac centers, with growing interest in both mitral and tricuspid applications.
India Transcatheter Edge-to-Edge Repair (TEER) Devices Market Insight
The India Transcatheter Edge-to-Edge Repair (TEER) devices market registered a growing share within Asia-Pacific in 2024, attributed to expanding tertiary cardiac centers, rising patient awareness of less invasive alternatives, and investments in interventional cardiology capacity. While the absolute base remains modest compared with developed markets, strong private hospital networks, increasing affordability, and targeted training initiatives are driving rapid uptake in urban centers. The push to decentralize advanced cardiac care and partnerships with global device manufacturers are key factors propelling the market.
Transcatheter Edge-to-Edge Repair (TEER) Devices Market Share
The Transcatheter Edge-to-Edge Repair (TEER) Devices industry is primarily led by well-established companies, including:
- Abbott (U.K.)
- Edwards Lifesciences Corporation (U.K.)
- Boston Scientific Corporation (U.K.)
- Medtronic (Ireland)
- LivaNova PLC (U.K.)
- MicroPort Scientific Corporation (China)
- JenaValve Technology, Inc. (Germany)
- Meril Life Sciences Pvt. Ltd. (India)
- CryoLife, Inc. (U.K.)
- W. L. Gore & Associates, Inc. (U.K.)
- Venus Medtech (Hangzhou) Inc. (China)
- Lepu Medical Technology Co., Ltd. (China)
- Braile Biomedica (Brazil)
- Transcatheter Technologies GmbH (Germany)
- CardiAQ Valve Technologies (U.K.)
- Colibri Heart Valve, LLC (U.K.)
- AtriCure, Inc. (U.K.)
- Xeltis AG (Switzerland)
- 4C Medical Technologies, Inc. (U.K.)
- Jenscare Scientific Co., Ltd. (China)
What are the Recent Developments in Global Transcatheter Edge-to-Edge Repair (TEER) Devices Market?
- In June 2025, Meril Life Sciences launched “MyClip”, India’s first indigenously developed TEER system for treating severe mitral regurgitation, unveiled during the Structural Heart Innovation event in Vapi, Gujarat. This minimally invasive device promises reduced hospital stays and broader access for high-risk populations
- In March 2025, Abbott presented two-year outcomes from the TRILUMINATE Pivotal Trial showing that the TriClip TEER system sustained tricuspid regurgitation (TR) reductions and quality-of-life improvements KCCQ gains remained beyond 15 points, and heart failure hospitalizations dropped by 28% compared to medical therapy
- In August 2024, the investigator-led Tri.fr trial reported that adding tricuspid TEER to optimized medical therapy significantly improved clinical outcomes and quality of life over 1 year—74% of patients met composite endpoints versus 41% with medical therapy alone
- In April 2024, the U.S. FDA approved Abbott’s TriClip G4 TEER system for treating tricuspid regurgitation in patients unsuitable for surgery. The decision, backed by favorable safety and efficacy data, opened percutaneous repair options for a previously underserved population
- In May 2021, a late-breaking study at the AATS meeting revealed that 95% of patients requiring surgery after failed MitraClip TEER needed full surgical mitral valve replacement, underscoring the importance of careful patient selection and informed consent in TEER procedures
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.