Market Analysis and Insights Global Transthyretin Amyloid Cardiomyopathy Treatment Market
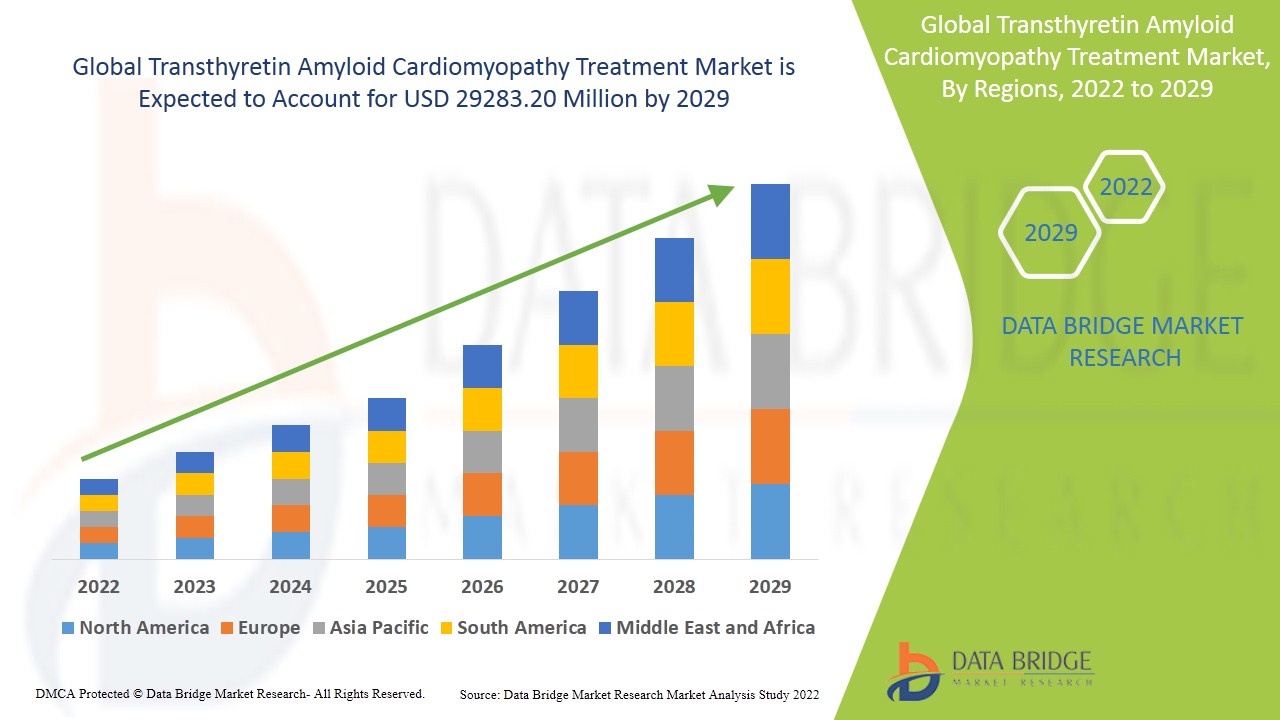
The transthyretin amyloid cardiomyopathy treatment market is expected to witness market growth at a rate of 25.1% in the forecast period of 2022 to 2029, and is estimated to reach the value of USD 29283.20 million by 2029. Data Bridge Market Research report on transthyretin amyloid cardiomyopathy treatment market provides analysis and insights regarding the various factors expected to be prevalent throughout the forecast period while providing their impacts on the market’s growth. The rise in healthcare sector globally is escalating the growth of transthyretin amyloid cardiomyopathy treatment market.
Transthyretin amyloid cardiomyopathy (ATTR-CM) refers to a form of transthyretin amyloidosis (ATTR) that tends to affect the heart. This condition leads to restrictive cardiomyopathy and progressive heart failure. The disorder is known to be a rare, underdiagnosed, and life-threatening disease and causes damage to the heart’s muscles.
The surge in the number of people suffering from transthyretin amyloid cardiomyopathy across the globe acts as one of the major factors driving the growth of transthyretin amyloid cardiomyopathy treatment market. The increase in demand for specific drugs for treating symptoms of the rare disorder and improvement in diagnostic procedures for better diagnosis accelerate the market growth. The increase in in number of clinical trials for enhancement of drugs and therapies, and high focus on research and development of transthyretin amyloid cardiomyopathy (ATTR-CM) treatment further influence the market. Additionally, rise in healthcare expenditure, research and development activities, growth in awareness and improvement of healthcare services positively affect the transthyretin amyloid cardiomyopathy treatment market. Furthermore, development of a novel drug products extend profitable opportunities to the market players in the forecast period of 2022 to 2029.
On the other hand, high cost associated with the treatment and lack of diagnosis are expected to obstruct the market growth. Lack of skilled professionals is projected to challenge the transthyretin amyloid cardiomyopathy treatment market in the forecast period of 2022-2029.
This transthyretin amyloid cardiomyopathy treatment market report provides details of new recent developments, trade regulations, import export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info transthyretin amyloid cardiomyopathy treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Transthyretin Amyloid Cardiomyopathy Treatment Market Scope and Market Size
The transthyretin amyloid cardiomyopathy treatment market is segmented on the basis of drug, indication, distribution channel and end-users. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- On the basis of product type, the transthyretin amyloid cardiomyopathy treatment market is segmented into inostersen, nonsteroidal anti-inflammatory drugs (NSAID), partisiran, tafamidis and others.
- On the basis of Indication, the transthyretin amyloid cardiomyopathy treatment market is segmented into wild type ATTR amyloidosis and hereditary ATTR amyloidosis.
- On the basis of distribution channel, the transthyretin amyloid cardiomyopathy treatment market is segmented into hospital pharmacy, retail pharmacy and online pharmacy.
- On the basis of end-users, the transthyretin amyloid cardiomyopathy treatment market is segmented into hospitals, homecare, specialty clinics and others.
Transthyretin Amyloid Cardiomyopathy Treatment Market Country Level Analysis
The transthyretin amyloid cardiomyopathy treatment market is analyzed and market size information is provided by country, drug, indication, distribution channel and end-users as referenced above.
The countries covered in the global transthyretin amyloid cardiomyopathy treatment market report are U.S., Canada and Mexico in North America, Peru, Brazil, Argentina and Rest of South America as part of South America, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Rest of Europe in Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia-Pacific (APAC) in Asia-Pacific (APAC), South Africa, Saudi Arabia, U.A.E, Kuwait, Israel, Egypt, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA).
North America dominates the transthyretin amyloid cardiomyopathy treatment market due to the increase in the number of diagnosis procedures and presence of leading manufacturers within the region. Asia-Pacific is expected to witness high growth during the forecast period of 2022 to 2029 because of the expansion of the pharmaceutical industry in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, disease epidemiology and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of sales channels are considered while providing forecast analysis of the country data.
Patient Epidemiology Analysis
The transthyretin amyloid cardiomyopathy treatment market also provides you with detailed market analysis for patient analysis, prognosis and cures. Prevalence, incidence, mortality, adherence rates are some of the data variables that are available in the report. Direct or indirect impact analysis of epidemiology to market growth are analyzed to create a more robust and cohort multivariate statistical model for forecasting the market in the growth period.
Competitive Landscape and Transthyretin Amyloid Cardiomyopathy Treatment Market Share Analysis
The transthyretin amyloid cardiomyopathy treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies’ focus related transthyretin amyloid cardiomyopathy treatment market.
Some of the major players operating in the transthyretin amyloid cardiomyopathy treatment market are Merck & Co., Inc., Pfizer Inc., AstraZeneca, Prothena Corporation plc., Ionis Pharmaceuticals, BELLUS Health Inc., Alnylam Pharmaceuticals, Inc.., Prothena, GlaxoSmithKline plc., Eidos Therapeutics, and SOM BIOTECH, among others.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER'S 5 FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 INCIDENCE OF ALL BY GENDER
5.2 TREATMENT RATE
5.3 MORTALITY RATE
5.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
5.5 PATEINT TREATMENT SUCCESS RATES
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH CARDIOLOGIST
6.8 OTHER KOL SNAPSHOTS
7 REGULATORY SCENARIO
8 PIPELINE ANALYSIS
8.1 CLINICAL TRIALS AND PHASE ANALYSIS
8.2 DRUG THERAPY PIPELINE
8.2.1 PRX-004
8.2.2 AG10
8.2.3 OTHERS
8.3 PHASE III CANDIDATES
8.4 PHASE II CANDIDATES
8.5 PHASE I CANDIDATES
8.6 OTHERS (PRE-CLINICAL AND RESEARCH)
9 MARKET OVERVIEW
9.1 DRIVERS
9.2 RESTRAINS
9.3 OPPURTUNITY
9.4 CHALLENGES
10 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY TYPE
10.1 OVERVIEW
10.2 FAMILIAL AMYLOIDOSIS (ATTRM)
10.2.1 ONPATTRO
10.2.2 TEGSEDI
10.2.3 VYNDAMAX
10.3 WILD-TYPE AMYLOIDOSIS (ATTRWT)
10.3.1 VYNDAQEL
11 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY TREATMENT
12 (NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS)
12.1 OVERVIEW
12.2 MEDICATION
12.2.1 ATTR SILENCERS
12.2.1.1. PATISIRAN
12.2.1.2. INOTERSEN
12.2.2 ATTR STABILIZERS
12.2.2.1. TAFAMIDIS
12.2.2.2. AG10
12.2.3 NONSTEROIDAL ANTI-INFLAMMATORY DRUG (NSAID)
12.2.3.1. DIFLUNSIAL
12.2.3.2. OTHERS
12.2.4 FIBRIL DISRUPTORS
12.2.4.1. DOXYCYCLINE
12.2.4.2. TAUROURSODEOXYCHOLIC ACID
12.2.5 ANTICOAGULANTS
12.2.5.1. INJECTABLE
12.2.5.1.1. WARFARIN
12.2.5.1.2. OTHERS
12.2.5.2. ORAL
12.2.5.2.1. APIXABAN
12.2.5.2.2. RIVAROXABAN
12.2.6 ANTIARRHYTHMICS
12.2.6.1. AMIODARONE
12.2.6.2. OTHERS
12.2.7 LOOP DIURETICS
12.2.7.1. TORSEMIDE
12.2.7.2. BUMETANIDE
12.3 TRANSPLANTATION
12.3.1 LIVER TRANSPLANTATION
12.3.1.1. MARKET VALUE (USD)
12.3.1.2. MARKET VOLUME (UNIT)
12.3.1.3. ASP (USD)
12.3.2 HEART TRANSPLANTATION
12.3.2.1. MARKET VALUE (USD)
12.3.2.2. MARKET VOLUME (UNIT)
12.3.2.3. ASP (USD)
12.4 PACEMAKERS
12.4.1 MARKET VALUE (USD)
12.4.2 MARKET VOLUME (UNIT)
12.4.3 ASP (USD)
13 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY PATIENT TYPE
13.1 OVERVIEW
13.2 CHILD
13.2.1 MALE
13.2.2 FEMALE
13.3 ADULT
13.3.1 MALE
13.3.2 FEMALE
13.4 GERIATRIC
13.4.1 MALE
13.4.2 FEMALE
14 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY DOSAGE FORM
14.1 OVERVIEW
14.2 ORAL
14.2.1 SOLID
14.2.1.1. TABLETS
14.2.1.2. CAPSULES
14.2.1.3. OTHERS
14.2.2 LIQUID
14.2.2.1. SOLUTIONS
14.2.2.2. SYRUPS
14.2.2.3. OTHERS
14.3 PARENTERAL
14.3.1 INFUSION
14.3.2 INTRAVENOUS
14.3.3 OTHERS
15 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITAL
15.3 CLINICS
15.4 HOME HEALTHCARE
15.5 SPECIALITY CENTER
15.6 AMBULTORY CENTERS
15.7 OTHERS
16 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 HOSPITAL PHARMACY
16.3 ONLINE PHARMACY
16.4 RETAIL PHARMACY
16.5 OTHERS
17 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY GEOGRAPHY
17.1 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
17.2 NORTH AMERICA
17.2.1 U.S.
17.2.1.1. U.S. TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY TYPE
17.2.1.2. U.S. TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY TREATMENT
17.2.1.3. U.S. TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY PATIENT TYPE
17.2.1.4. U.S. TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY DOSAGE FORM
17.2.1.5. U.S. TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY END USER
17.2.1.6. U.S. TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL
17.2.2 CANADA
17.2.3 MEXICO
17.3 EUROPE
17.3.1 GERMANY
17.3.2 FRANCE
17.3.3 U.K.
17.3.4 HUNGARY
17.3.5 LITHUANIA
17.3.6 AUSTRIA
17.3.7 IRELAND
17.3.8 NORWAY
17.3.9 POLAND
17.3.10 ITALY
17.3.11 SPAIN
17.3.12 RUSSIA
17.3.13 TURKEY
17.3.14 NETHERLANDS
17.3.15 SWITZERLAND
17.3.16 REST OF EUROPE
17.4 ASIA-PACIFIC
17.4.1 JAPAN
17.4.2 CHINA
17.4.3 SOUTH KOREA
17.4.4 INDIA
17.4.5 AUSTRALIA
17.4.6 SINGAPORE
17.4.7 THAILAND
17.4.8 MALAYSIA
17.4.9 INDONESIA
17.4.10 PHILIPPINES
17.4.11 VIETNAM
17.4.12 REST OF ASIA-PACIFIC
17.5 SOUTH AMERICA
17.5.1 BRAZIL
17.5.2 ARGENTINA
17.5.3 PERU
17.5.4 REST OF SOUTH AMERICA
17.6 MIDDLE EAST AND AFRICA
17.6.1 SOUTH AFRICA
17.6.2 SAUDI ARABIA
17.6.3 UAE
17.6.4 EGYPT
17.6.5 KUWAIT
17.6.6 ISRAEL
17.6.7 REST OF MIDDLE EAST AND AFRICA
17.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
18 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, SWOT AND DBMR ANALYSIS
19 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, COMPANY LANDSCAPE
19.1 COMPANY SHARE ANALYSIS: GLOBAL
19.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
19.3 COMPANY SHARE ANALYSIS: EUROPE
19.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
19.5 MERGERS & ACQUISITIONS
19.6 NEW PRODUCT DEVELOPMENT & APPROVALS
19.7 EXPANSIONS
19.8 REGULATORY CHANGES
19.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
20 GLOBAL TRANSTHYRETIN AMYLOID CARDIOMYOPATHY TREATMENT MARKET, COMPANY PROFILE
20.1 ALNYLAM PHARMACEUTICALS, INC
20.1.1 COMPANY OVERVIEW
20.1.2 REVENUE ANALYSIS
20.1.3 GEOGRAPHIC PRESENCE
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.2 AKCEA THERAPEUTICS
20.2.1 COMPANY OVERVIEW
20.2.2 REVENUE ANALYSIS
20.2.3 GEOGRAPHIC PRESENCE
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 PFIZER INC.
20.3.1 COMPANY OVERVIEW
20.3.2 REVENUE ANALYSIS
20.3.3 GEOGRAPHIC PRESENCE
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENTS
20.4 ARTI INDUSTRIES LTD
20.4.1 COMPANY OVERVIEW
20.4.2 REVENUE ANALYSIS
20.4.3 GEOGRAPHIC PRESENCE
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENTS
20.5 ENOMARK
20.5.1 COMPANY OVERVIEW
20.5.2 REVENUE ANALYSIS
20.5.3 GEOGRAPHIC PRESENCE
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENTS
20.6 MAYNE PHARMA
20.6.1 COMPANY OVERVIEW
20.6.2 REVENUE ANALYSIS
20.6.3 GEOGRAPHIC PRESENCE
20.6.4 PRODUCT PORTFOLIO
20.6.5 RECENT DEVELOPMENTS
20.7 FISHER SCIENTIFIC
20.7.1 COMPANY OVERVIEW
20.7.2 REVENUE ANALYSIS
20.7.3 GEOGRAPHIC PRESENCE
20.7.4 PRODUCT PORTFOLIO
20.7.5 RECENT DEVELOPMENTS
20.8 AMIRALL
20.8.1 COMPANY OVERVIEW
20.8.2 REVENUE ANALYSIS
20.8.3 GEOGRAPHIC PRESENCE
20.8.4 PRODUCT PORTFOLIO
20.8.5 RECENT DEVELOPMENTS
20.9 TEVA PHARMACEUTICAL USA, INC
20.9.1 COMPANY OVERVIEW
20.9.2 REVENUE ANALYSIS
20.9.3 GEOGRAPHIC PRESENCE
20.9.4 PRODUCT PORTFOLIO
20.9.5 RECENT DEVELOPMENTS
20.1 LUPIN PHARMACEUTICAL, INC
20.10.1 COMPANY OVERVIEW
20.10.2 REVENUE ANALYSIS
20.10.3 GEOGRAPHIC PRESENCE
20.10.4 PRODUCT PORTFOLIO
20.10.5 RECENT DEVELOPMENTS
20.11 UPSHER-SMITH LABORATORIES, LLC
20.11.1 COMPANY OVERVIEW
20.11.2 REVENUE ANALYSIS
20.11.3 GEOGRAPHIC PRESENCE
20.11.4 PRODUCT PORTFOLIO
20.11.5 RECENT DEVELOPMENTS
20.12 BRISTOL MEYERS SQUIBB COMPANY
20.12.1 COMPANY OVERVIEW
20.12.2 REVENUE ANALYSIS
20.12.3 GEOGRAPHIC PRESENCE
20.12.4 PRODUCT PORTFOLIO
20.12.5 RECENT DEVELOPMENTS
20.13 DR REDDY’S LABORATORIES LTD
20.13.1 COMPANY OVERVIEW
20.13.2 REVENUE ANALYSIS
20.13.3 GEOGRAPHIC PRESENCE
20.13.4 PRODUCT PORTFOLIO
20.13.5 RECENT DEVELOPMENTS
20.14 BAYER AG
20.14.1 COMPANY OVERVIEW
20.14.2 REVENUE ANALYSIS
20.14.3 GEOGRAPHIC PRESENCE
20.14.4 PRODUCT PORTFOLIO
20.14.5 RECENT DEVELOPMENTS
20.15 CAMBER PHARMACEUTICALS
20.15.1 COMPANY OVERVIEW
20.15.2 REVENUE ANALYSIS
20.15.3 GEOGRAPHIC PRESENCE
20.15.4 PRODUCT PORTFOLIO
20.15.5 RECENT DEVELOPMENTS
20.16 SARFEZ
20.16.1 COMPANY OVERVIEW
20.16.2 REVENUE ANALYSIS
20.16.3 GEOGRAPHIC PRESENCE
20.16.4 PRODUCT PORTFOLIO
20.16.5 RECENT DEVELOPMENTS
20.17 MEDTRONIC
20.17.1 COMPANY OVERVIEW
20.17.2 REVENUE ANALYSIS
20.17.3 GEOGRAPHIC PRESENCE
20.17.4 PRODUCT PORTFOLIO
20.17.5 RECENT DEVELOPMENTS
20.18 ABBOTT
20.18.1 COMPANY OVERVIEW
20.18.2 REVENUE ANALYSIS
20.18.3 GEOGRAPHIC PRESENCE
20.18.4 PRODUCT PORTFOLIO
20.18.5 RECENT DEVELOPMENTS
20.19 BOSTON SCIENTIC CORPORATION
20.19.1 COMPANY OVERVIEW
20.19.2 REVENUE ANALYSIS
20.19.3 GEOGRAPHIC PRESENCE
20.19.4 PRODUCT PORTFOLIO
20.19.5 RECENT DEVELOPMENTS
20.2 OSCOR INC
20.20.1 COMPANY OVERVIEW
20.20.2 REVENUE ANALYSIS
20.20.3 GEOGRAPHIC PRESENCE
20.20.4 PRODUCT PORTFOLIO
20.20.5 RECENT DEVELOPMENTS
20.21 BIOTRINIK
20.21.1 COMPANY OVERVIEW
20.21.2 REVENUE ANALYSIS
20.21.3 GEOGRAPHIC PRESENCE
20.21.4 PRODUCT PORTFOLIO
20.21.5 RECENT DEVELOPMENTS
20.22 MICROPORT SCIENTIFIC CORPORATION
20.22.1 COMPANY OVERVIEW
20.22.2 REVENUE ANALYSIS
20.22.3 GEOGRAPHIC PRESENCE
20.22.4 PRODUCT PORTFOLIO
20.22.5 RECENT DEVELOPMENTS
20.23 OSCOR INC
20.23.1 COMPANY OVERVIEW
20.23.2 REVENUE ANALYSIS
20.23.3 GEOGRAPHIC PRESENCE
20.23.4 PRODUCT PORTFOLIO
20.23.5 RECENT DEVELOPMENTS
20.24 PACETRONIX
20.24.1 COMPANY OVERVIEW
20.24.2 PRODUCT PORTFOLIO
20.24.3 REVENUE ANALYSIS
20.24.4 GEOGRAPHIC PRESENCE
20.24.5 PRODUCT PORTFOLIO
20.25 MEDICO S.R.L
20.25.1 COMPANY OVERVIEW
20.25.2 PRODUCT PORTFOLIO
20.25.3 REVENUE ANALYSIS
20.25.4 GEOGRAPHIC PRESENCE
20.25.5 PRODUCT PORTFOLIO
20.26 SUN PHARMACEUTICAL PVT LTD
20.26.1 COMPANY OVERVIEW
20.26.2 PRODUCT PORTFOLIO
20.26.3 REVENUE ANALYSIS
20.26.4 GEOGRAPHIC PRESENCE
20.26.5 PRODUCT PORTFOLIO
20.27 LEPU MEDICAL TECHNOLOGY
20.27.1 COMPANY OVERVIEW
20.27.2 PRODUCT PORTFOLIO
20.27.3 REVENUE ANALYSIS
20.27.4 GEOGRAPHIC PRESENCE
20.27.5 PRODUCT PORTFOLIO
20.28 LIVANOVA PLC
20.28.1 COMPANY OVERVIEW
20.28.2 PRODUCT PORTFOLIO
20.28.3 REVENUE ANALYSIS
20.28.4 GEOGRAPHIC PRESENCE
20.28.5 PRODUCT PORTFOLIO
20.29 MEDICO S.R.L
20.29.1 COMPANY OVERVIEW
20.29.2 PRODUCT PORTFOLIO
20.29.3 REVENUE ANALYSIS
20.29.4 GEOGRAPHIC PRESENCE
20.29.5 PRODUCT PORTFOLIO
20.3 SUN PHARMACEUTICALS PVT LTD
20.30.1 COMPANY OVERVIEW
20.30.2 PRODUCT PORTFOLIO
20.30.3 REVENUE ANALYSIS
20.30.4 GEOGRAPHIC PRESENCE
20.30.5 PRODUCT PORTFOLIO
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
21 RELATED REPORTS
22 CONCLUSION
23 QUESTIONNAIRE
24 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.