Global Tumor Lysis Syndrome Market
Market Size in USD Billion
CAGR :
% 
 USD
2.30 Billion
USD
4.79 Billion
2024
2032
USD
2.30 Billion
USD
4.79 Billion
2024
2032
| 2025 –2032 | |
| USD 2.30 Billion | |
| USD 4.79 Billion | |
|
|
|
|
Tumor Lysis Syndrome Market Size
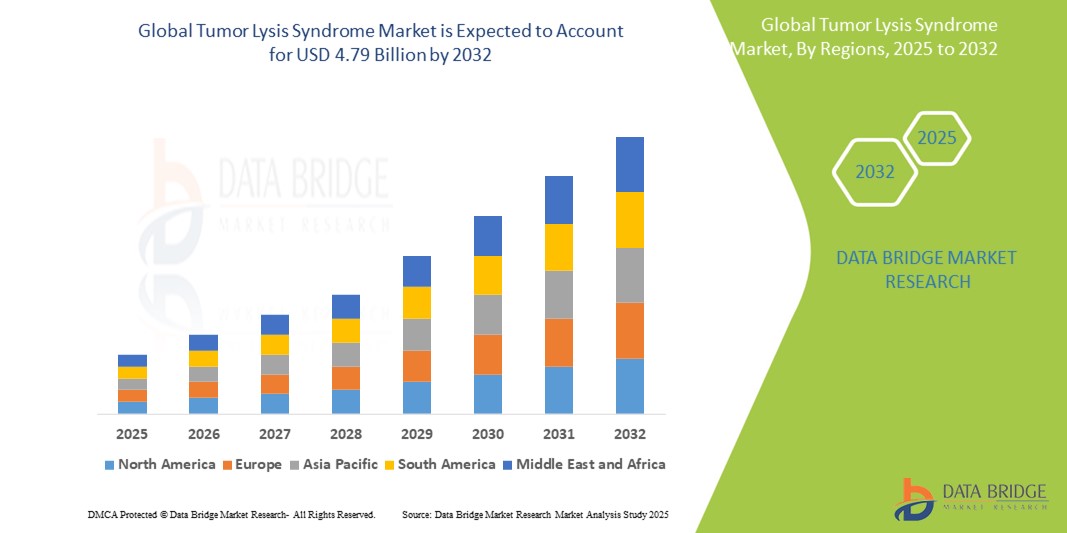
- The Global Tumor Lysis Syndrome Market size was valued at USD 2.30 billion in 2024 and is expected to reach USD 4.79 billion by 2032, at a CAGR of 9.6% during the forecast period
- The market growth is primarily driven by the rising incidence of hematologic malignancies and solid tumors treated with aggressive therapies such as chemotherapy, radiation, and biological treatments. These interventions increase the risk of TLS, thereby boosting demand for effective preventive and treatment strategies
- Moreover, increasing awareness among clinicians, the availability of advanced diagnostic tools like serum electrolyte tests and creatinine assays, and the growing use of uric acid-lowering drugs such as allopurinol and rasburicase are accelerating the market expansion. The development of targeted therapies and improved clinical guidelines for TLS management are further strengthening the market outlook
Tumor Lysis Syndrome Market Analysis
- Tumor Lysis Syndrome (TLS), a life-threatening oncological emergency resulting from rapid tumor cell breakdown, is gaining increased clinical attention due to its growing incidence among patients undergoing chemotherapy, radiation therapy, and targeted biological treatments—especially in hematologic malignancies such as leukemia and lymphoma.
- The escalating demand for effective TLS management is primarily driven by the rising global cancer burden, increasing use of aggressive cancer therapies, and the need for early diagnosis and prevention of complications like hyperuricemia and acute kidney injury.
- North America dominates the tumor lysis syndrome market with the largest revenue share of over 40.5% in 2025, attributed to high cancer prevalence, robust healthcare infrastructure, early adoption of advanced therapeutics (e.g., rasburicase), and strong clinical awareness. The U.S. leads in TLS treatment advancements due to widespread access to diagnostics and supportive care protocols.
- Asia-Pacific is expected to be the fastest-growing region in the tumor lysis syndrome market during the forecast period, driven by increasing oncology patient volumes, expanding healthcare access, and government initiatives to improve cancer care in countries such as China and India.
- Asia-Pacific is expected to be the fastest growing region in the Tumor Lysis Syndrome market during the forecast period due to increasing urbanization and rising disposable incomes
- Among drug types, the rasburicase segment is expected to dominate the market with a significant share of 45.2% in 2025, owing to its rapid and effective uric acid reduction capabilities in high-risk TLS cases, as well as favorable clinical guidelines supporting its use over traditional agents like allopurinol.
Report Scope and Tumor Lysis Syndrome Market Segmentation
|
Attributes |
Tumor Lysis Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Tumor Lysis Syndrome Market Trends
“Rising Focus on Early Risk Stratification and Prophylactic Management”
- A significant and growing trend in the global Tumor Lysis Syndrome (TLS) market is the increasing emphasis on early identification of high-risk patients and the adoption of proactive prophylactic treatment. Healthcare providers are integrating TLS risk assessment protocols into standard oncology practice, particularly for hematologic malignancies like acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma, where TLS incidence is higher.
- For instance, the implementation of the Cairo-Bishop classification and other TLS risk stratification tools in clinical workflows has enabled oncologists to pre-emptively identify patients who may require prophylaxis with urate-lowering agents such as rasburicase or febuxostat, rather than waiting for overt TLS to manifest.
- Additionally, real-time monitoring through serum creatinine, uric acid, phosphate, and potassium levels is being supported by automated hospital systems, helping providers initiate timely intervention before complications such as acute kidney injury arise.
- Pharmaceutical companies are responding to this trend by investing in education campaigns, clinical decision support tools, and broader indications for their TLS-focused drugs. For instance, several drugmakers are supporting the inclusion of TLS prophylaxis in cancer care pathways across both developed and developing markets.
- This trend of preemptive care, improved diagnostic practices, and clinical vigilance is fundamentally changing the TLS treatment paradigm from reactive to preventive. As a result, market players focusing on early-intervention therapies and integrated monitoring solutions are likely to gain competitive advantages.
Tumor Lysis Syndrome Market Dynamics
Driver
“Rising Cancer Incidence and Growing Use of Aggressive Oncology Therapies”
- The rising global burden of cancer, particularly blood cancers such as leukemia and lymphoma, is a key driver of the Tumor Lysis Syndrome market. With oncology care increasingly involving high-efficacy therapies like intensive chemotherapy, monoclonal antibodies, and CAR-T cell therapy, the incidence of treatment-induced TLS is rising sharply.
- For instance, the growing adoption of CAR-T cell therapy in relapsed/refractory leukemia and lymphoma patients has significantly increased TLS risk due to the rapid destruction of malignant cells. To mitigate these risks, guidelines such as those by the American Society of Clinical Oncology (ASCO) recommend pre-treatment with urate-lowering agents in high-risk individuals.
- The growing availability and reimbursement of TLS treatments like rasburicase in developed markets and the inclusion of TLS risk management protocols in cancer care pathways globally are supporting widespread market growth.
- Additionally, hospital protocols now emphasize early hospitalization and fluid management for high-risk patients, reinforcing the need for TLS-specific therapeutics and diagnostics.
- As cancer care becomes more personalized and intensive, the demand for TLS prevention and management solutions is expected to grow significantly across hospitals, specialty clinics, and home care settings.
Restraint/Challenge
“High Drug Costs and Limited Access in Low-Resource Settings”
- One of the major challenges for the global TLS market is the high cost of key drugs such as rasburicase, which can limit their accessibility, especially in low- and middle-income countries. Despite its clinical efficacy, rasburicase remains out of reach for many healthcare systems due to limited availability, budget constraints, and lack of insurance coverage.
- In many developing regions, clinicians rely on less effective alternatives like allopurinol, which may delay treatment response and increase the risk of complications. Moreover, awareness regarding TLS and its management protocols remains low in several regions, leading to underdiagnosis and delayed intervention.
- Another challenge is the lack of standardized TLS screening and treatment protocols in many cancer treatment centers, especially outside major urban hospitals.
- To address these barriers, companies are exploring biosimilar development, tiered pricing strategies, and partnership models with governments and NGOs to expand access to life-saving therapies.
- However, unless cost-effective treatment options and improved clinical education efforts are implemented on a global scale, TLS outcomes will continue to show disparity between high-income and low-income regions, thereby restraining uniform market growth.
Tumor Lysis Syndrome Market Scope
The market is segmented on the basis of pathophysiology, causative therapy, diagnosis, drug type, route of administration, end user, and distribution channel.
- By Pathophysiology
On the basis of pathophysiology, the TLS market is segmented into hyperuricemia, acute kidney injury, and others. The hyperuricemia segment holds the largest market revenue share of approximately 47.3% in 2025, driven by the fact that elevated uric acid levels are among the earliest and most common manifestations of TLS. The clinical emphasis on managing hyperuricemia to prevent further complications like renal failure has led to widespread use of urate-lowering agents such as rasburicase and allopurinol, making this segment the most dominant in terms of demand and therapeutic focus.
The acute kidney injury (AKI) segment is projected to witness the fastest CAGR of 10.6% from 2025 to 2032, as AKI is a serious and life-threatening consequence of TLS. Increasing awareness, improved monitoring tools, and advancements in diagnostic markers for early renal dysfunction are fueling demand for prompt intervention and targeted therapies to manage kidney-related TLS outcomes, especially in critical care and high-risk oncology patients.
• By Causative Therapy
On the basis of causative therapy, the market is segmented into chemotherapy, radiation therapy, biological therapy, and others. The chemotherapy segment dominates the market in 2025, as it remains the most common trigger for TLS, particularly in hematologic malignancies. Intensive chemotherapy regimens are known to induce rapid tumor cell lysis, thus necessitating preventive and therapeutic interventions for TLS.
The biological therapy segment is expected to grow at the fastest rate from 2025 to 2032. The increasing use of immunotherapy and targeted therapies like CAR-T cells has raised new clinical considerations for TLS risk management, as these therapies can induce swift tumor cell death in relapsed or refractory cases, prompting heightened need for proactive TLS control.
• By Diagnosis
The TLS market by diagnosis is segmented into blood urea nitrogen test, creatinine test, serum electrolytes test, and others. The serum electrolytes test segment is expected to hold the largest revenue share in 2025 due to its critical role in identifying hallmark metabolic imbalances like hyperkalemia, hyperphosphatemia, and hypocalcemia. These tests are vital in both TLS risk assessment and ongoing monitoring.
The creatinine test segment is anticipated to witness the fastest CAGR from 2025 to 2032, as elevated serum creatinine is an early indicator of renal dysfunction, a major TLS complication. Increased emphasis on early renal damage detection is driving its usage across oncology settings.
• By Drug Type
By drug type, the market is segmented into allopurinol, rasburicase, febuxostat, and others. The rasburicase segment is expected to dominate the market with the highest revenue share of approximately 45.2% in 2025, due to its superior efficacy in rapidly lowering uric acid levels and its strong recommendation in international TLS guidelines for high-risk patients.
The febuxostat segment is projected to grow at the fastest CAGR during the forecast period. As an alternative xanthine oxidase inhibitor for patients intolerant to allopurinol, febuxostat is gaining traction due to its improved safety profile and growing availability in emerging markets.
• By Route of Administration
The market is segmented by route of administration into oral, injectable, and others. The injectable segment holds the largest share in 2025, primarily due to the intravenous administration of rasburicase and other emergency treatments for high-risk TLS cases. Injectable therapies offer rapid systemic action, which is crucial in acute care settings.
The oral segment is expected to witness the fastest growth from 2025 to 2032, as drugs like allopurinol and febuxostat are widely used for outpatient TLS prophylaxis, particularly in low- to moderate-risk patients.
• By End User
Based on end user, the TLS market is segmented into hospitals, homecare, specialty clinics, and others. The hospital segment leads the market in 2025, driven by the high concentration of oncology treatment centers, inpatient TLS management protocols, and access to intensive care facilities for critical cases.
The homecare segment is projected to witness the fastest CAGR during the forecast period. This growth is fueled by the expansion of oncology home infusion services and the increasing adoption of oral prophylaxis therapies, enabling some elements of TLS management to transition to outpatient or home settings.
• By Distribution Channel
The market is segmented by distribution channel into hospital pharmacy, online pharmacy, retail pharmacy, and others. The hospital pharmacy segment dominates the market in 2025, supported by direct supply chains for injectable drugs and immediate availability in acute care environments.
The online pharmacy segment is anticipated to register the fastest CAGR from 2025 to 2032, with the rise of digital healthcare platforms, improved e-pharmacy regulations, and convenience of access to oral TLS medications playing a key role in expanding this channel.
Tumor Lysis Syndrome Market Regional Analysis
- North America dominates the tumor lysis syndrome market with the largest revenue share of 40.5% in 2025, driven by a high prevalence of hematologic malignancies, early adoption of advanced oncology therapies, and strong clinical awareness regarding TLS prevention and management.
- The region benefits from well-established healthcare infrastructure, widespread access to effective TLS therapeutics such as rasburicase and febuxostat, and the presence of leading pharmaceutical players actively involved in research and distribution of TLS-specific treatments.
- Additionally, favorable reimbursement policies, integration of TLS risk management into oncology care pathways, and increasing adoption of CAR-T and immunotherapy treatments—especially in the U.S.—are further fueling market growth. These factors collectively position North America as the most mature and lucrative regional market for TLS solutions across hospital, specialty clinic, and homecare settings.
U.S. Tumor Lysis Syndrome Market Insight
The U.S. tumor lysis syndrome market captured the largest revenue share of approximately 78% within North America in 2025, driven by a high prevalence of hematologic malignancies, advanced cancer care infrastructure, and widespread adoption of intensive therapies such as CAR-T, immunotherapy, and aggressive chemotherapy. The integration of TLS risk stratification and prophylaxis into clinical guidelines by bodies such as ASCO is further fueling market demand. In addition, the availability of advanced diagnostic tools and broad access to high-cost treatments like rasburicase support strong market penetration across hospitals, specialty oncology centers, and outpatient infusion facilities.
Europe Tumor Lysis Syndrome Market Insight
The European tumor lysis syndrome market is projected to expand at a solid CAGR during the forecast period, primarily driven by improved oncology care standards, increasing adoption of targeted cancer therapies, and rising awareness of TLS risk. The expansion of national cancer programs, along with reimbursement support for urate-lowering drugs, is fostering market growth across major European countries. Additionally, strong pharmaceutical R&D capabilities and access to trained healthcare professionals are reinforcing early diagnosis and TLS management protocols across inpatient and outpatient settings.
U.K. Tumor Lysis Syndrome Market Insight
The U.K. tumor lysis syndrome market is expected to grow at a notable CAGR over the forecast period, supported by a rising incidence of hematologic malignancies and the expansion of the NHS cancer treatment portfolio. Increasing use of biologics and targeted therapies in both adult and pediatric oncology is elevating the focus on TLS prevention and early intervention. Enhanced awareness campaigns, clinical education, and access to cost-effective oral urate-lowering drugs are helping bridge treatment gaps, while national initiatives continue to improve access to oncology services.
Germany Tumor Lysis Syndrome Market Insight
Germany’s tumor lysis syndrome market is forecasted to grow steadily, driven by its highly developed healthcare infrastructure, strong focus on oncology innovation, and adoption of TLS-specific treatment protocols in tertiary care hospitals. The widespread availability of high-precision diagnostics and a robust base of practicing hematologists/oncologists are further boosting demand. Germany’s emphasis on clinical guideline adherence, including routine screening for TLS risk and prophylactic therapy administration, is enabling effective market expansion across public and private institutions.
Asia-Pacific Tumor Lysis Syndrome Market Insight
The Asia-Pacific TLS market is expected to grow at the fastest CAGR of over 11% in 2025, driven by increasing cancer incidence, expanding healthcare access, and improved availability of oncology therapies in countries like China, India, and Japan. Government-led initiatives for cancer care improvement, rising investments in hospital infrastructure, and the inclusion of TLS management in oncology protocols are accelerating market development. In addition, the growing number of specialty cancer centers and the emergence of local drug manufacturers are making TLS treatment more accessible and affordable in the region.
Japan Tumor Lysis Syndrome Market Insight
The Japan TLS market is experiencing sustained growth, supported by the country’s aging population, high prevalence of hematologic cancers, and established precision medicine initiatives. Japan’s oncology care system increasingly emphasizes early detection and risk management of TLS, particularly in patients undergoing intensive therapies. The presence of domestic pharmaceutical giants and a strong clinical research environment ensures access to both branded and generic TLS drugs, while integration with national electronic health records supports better TLS monitoring and adherence.
China Tumor Lysis Syndrome Market Insight
China accounted for the largest market revenue share in Asia-Pacific in 2025, fueled by its rapidly growing cancer patient population, urbanization, and healthcare reforms aimed at improving oncology treatment access. The country is seeing increasing adoption of high-efficacy therapies such as chemotherapy and immunotherapy, which heightens the risk of TLS and thus the demand for effective preventive treatments. The availability of affordable generics, growing investment in hospital infrastructure, and the expansion of national health insurance coverage are key contributors to market growth. China also hosts a robust network of domestic pharmaceutical companies that are increasingly entering the TLS space.
Tumor Lysis Syndrome Market Share
The Tumor Lysis Syndrome industry is primarily led by well-established companies, including:
- Johnson & Johnson Private Limited (U.S.)
- Ironwood Pharmaceuticals, Inc. (U.S.)
- Sanofi (France)
- The Menarini Group (Italy)
- Merck KGaA (Germany)
- Takeda Pharmaceutical Company Limited (Japan)
- AstraZeneca (U.K.)
- Hikma Pharmaceuticals PLC (U.K.)
- Pfizer Inc. (U.S.)
- Lonza (Switzerland)
- Amgen Inc. (U.S.)
- Genentech, Inc. (U.S.) (subsidiary of Roche, Switzerland)
- Ionis Pharmaceuticals (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- AbbVie Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Alexion Pharmaceuticals, Inc. (U.S.) (a subsidiary of AstraZeneca, U.K.)
- Mallinckrodt (Ireland)
- F. Hoffmann-La Roche Ltd (Switzerland)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL TUMOR LYSIS SYNDROME MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL TUMOR LYSIS SYNDROME MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL TUMOR LYSIS SYNDROME MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 EPIDEMIOLOGY
11.1 INCIDENCE OF ALL BY GENDER
11.2 TREATMENT RATE
11.3 MORTALITY RATE
11.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
11.5 PATIENT TREATMENT SUCCESS RATES
12 REGULATORY COMPLIANCE
12.1 REGULATORY AUTHORITIES
12.2 REGULATORY CLASSIFICATIONS
12.2.1 CLASS I
12.2.2 CLASS II
12.2.3 CLASS III
12.3 REGULATORY SUBMISSIONS
12.4 INTERNATIONAL HARMONIZATION
12.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
12.6 REGULATORY CHALLENGES AND STRATEGIES
13 PIPELINE ANALYSIS
13.1 CLINICAL TRIALS AND PHASE ANALYSIS
13.2 DRUG THERAPY PIPELINE
13.3 PHASE III CANDIDATES
13.4 PHASE II CANDIDATES
13.5 PHASE I CANDIDATES
13.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR TUMOR LYSIS SYNDROME MARKET
Company Name Product Name
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE FOR TUMOR LYSIS SYNDROME MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved but Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE FOR TUMOR LYSIS SYNDROME MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE FOR TUMOR LYSIS SYNDROME MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR TUMOR LYSIS SYNDROME MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
14 REIMBURSEMENT FRAMEWORK
15 OPPUTUNITY MAP ANALYSIS
16 VALUE CHAIN ANALYSIS
17 HEALTHCARE ECONOMY
17.1 HEALTHCARE EXPENDITURE
17.2 CAPITAL EXPENDITURE
17.3 CAPEX TRENDS
17.4 CAPEX ALLOCATION
17.5 FUNDING SOURCES
17.6 INDUSTRY BENCHMARKS
17.7 GDP RATION IN OVERALL GDP
17.8 HEALTHCARE SYSTEM STRUCTURE
17.9 GOVERNMENT POLICIES
17.1 ECONOMIC DEVELOPMENT
18 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY TREATMENT
18.1 OVERVIEW
18.2 MEDICATIONS
18.2.1 MARKET SIZE
18.2.1.1. MARKET VALUE (USD MN)
18.2.1.2. MARKET VOLUME (MILLION)
18.2.1.3. ASP (USD)
18.2.2 URICOSURIC AGENTS
18.2.2.1. ALLOPURINOL
18.2.2.1.1. BY BRAND
18.2.2.1.1.1 ZYLOPRIM
18.2.2.1.1.2 ALOPRIM
18.2.2.1.2. BY STRENGTH
18.2.2.1.2.1 100MG
18.2.2.1.2.2 300MG
18.2.2.1.2.3 500MG
18.2.2.2. RASBURICASE/ELITEK
18.2.2.2.1. 1.5MG
18.2.2.2.2. 7.5MG
18.2.3 ELECTROLYTES
18.2.3.1. DEXTROSE (D-GLUCOSE) PLUS INSULIN
18.2.3.1.1. BY TYPE
18.2.3.1.1.1 D50W
18.2.3.1.1.2 DGLUCOSE
18.2.3.1.1.3 GLUCOSE
18.2.3.1.2. BY CONCENTRATION
18.2.3.1.2.1 0.025
18.2.3.1.2.2 0.05
18.2.3.1.2.3 0.1
18.2.3.1.2.4 0.2
18.2.3.1.2.5 OTHERS
18.2.3.2. OTHERS
18.2.4 DIURETICS, LOOP
18.2.4.1. FUROSEMIDE
18.2.4.1.1. BY TYPE
18.2.4.1.1.1 LASIX
18.2.4.1.1.2 FUROSCIX
18.2.4.1.2. BY STRENGTH
18.2.4.1.2.1 20MG
18.2.4.1.2.2 40MG
18.2.4.1.2.3 80MG
18.2.4.1.2.4 OTHERS
18.2.4.2. OTHERS
18.2.5 ALKALINIZING AGENTS
18.2.5.1. ACETAZOLAMIDE/DIAMOX
18.2.5.1.1. 125MG
18.2.5.1.2. 250MG
18.2.5.1.3. 500MG
18.2.5.2. SODIUM BICARBONATE/NEUT
18.2.5.2.1. BY CONCENTRATION
18.2.5.2.1.1 0.04
18.2.5.2.1.2 0.042
18.2.5.2.1.3 0.075
18.2.5.2.1.4 0.084
18.2.5.2.2. BY STRENGTH
18.2.5.2.2.1 325MG
18.2.5.2.2.2 650MG
18.2.5.3. OTHERS
18.2.6 ELECTROLYTE SUPPLEMENTS, PARENTERAL
18.2.6.1. CALCIUM GLUCONATE
18.2.6.1.1. 50MG
18.2.6.1.2. 500MG
18.2.6.1.3. 650MG
18.2.6.2. CALCIUM CHLORIDE
18.2.6.3. OTHERS
18.2.7 ANTIDOTES, OTHER
18.2.7.1. SODIUM POLYSTYRENE SULFONATE
18.2.7.1.1. SPS
18.2.7.1.2. KAYEXALATE
18.2.7.1.3. KIONEX
18.2.7.1.4. KALEXATE
18.2.7.2. ALUMINUM HYDROXIDE
18.2.7.2.1. ALTERNAGEL
18.2.7.2.2. AMPHOJEL
18.2.7.2.3. NEPHROX
18.2.7.3. SEVELAMER HYDROCHLORIDE
18.2.7.3.1. BY TYPE
18.2.7.3.1.1 RENAGEL
18.2.7.3.1.2 RENVELA
18.2.7.3.2. BY STRENGTH
18.2.7.3.2.1 400MG
18.2.7.3.2.2 800MG
18.2.7.3.2.3 2400MG
18.2.7.4. FEBUXOSTAT
18.2.7.5. OTHERS
18.3 DIALYSIS
18.3.1 MARKET VALUE (USD MN)
18.3.2 MARKET VOLUME (MILLION)
18.3.3 ASP (USD)
18.4 OTHERS
19 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY ROUTE OF ADMINISTRATION
19.1 OVERVIEW
19.2 ORAL
19.2.1 TABLET
19.2.2 CAPSULE
19.2.3 SOLUTION
19.2.4 OTHERS
19.3 PARENTERAL
19.3.1 INTRAVENEOUS
19.3.2 INTRAMUSCULAR
19.3.3 SUBCUTANEOUS
19.4 OTHERS
20 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY DRUG TYPE
20.1 OVERVIEW
20.2 BRANDED
20.2.1 ZYLOPRIM
20.2.2 LOPURIN
20.2.3 ELITEK
20.2.4 OTHERS
20.3 GENERICS
21 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY PRESCRIPTION MODE
21.1 OVERVIEW
21.2 OTC DRUG
21.3 PRESCRIPTION DRUG
22 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY GENDER
22.1 OVERVIEW
22.2 MALE
22.2.1 PEDIATRIC
22.2.2 ADULT
22.2.3 GERIATRIC
22.3 FEMALE
22.3.1 PEDIATRIC
22.3.2 ADULT
22.3.3 GERIATRIC
23 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY AGE GROUP
23.1 OVERVIEW
23.2 BELOW 30 YEARS
23.3 31-60 YEARS
23.4 ABOVE 60 YEARS
24 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY INDICATION
24.1 OVERVIEW
24.2 NUMBNESS
24.3 SEIZURES
24.4 PARALYSIS
24.5 HEART PALPITATIONS
24.6 IRREGULAR HEARTBEATS
24.7 FLICKERING, BLURRED, OR DOUBLE VISION
24.8 UNCONTROLLABLE BODY MOVEMENTS
24.9 OTHERS
25 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY END USER
25.1 OVERVIEW
25.2 HOSPITAL
25.2.1 PRIVATE
25.2.2 PUBLIC
25.3 SPECIALTY CLINICS
25.4 HOME HEALTHCARE
25.5 CANCER RESEARCH INSTITUTE
25.6 OTHERS
26 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY DISTRIBUTION CHANNEL
26.1 OVERVIEW
26.2 DIRECT TENDER
26.3 RETAIL SALES
26.3.1 HOSPITAL PHARMACY
26.3.2 ONLINE PHARMACY
26.3.3 MEDICINE STORES
26.4 OTHERS
27 GLOBAL TUMOR LYSIS SYNDROME MARKET, COMPANY LANDSCAPE
27.1 COMPANY SHARE ANALYSIS: GLOBAL
27.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
27.3 COMPANY SHARE ANALYSIS: EUROPE
27.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
27.5 MERGERS & ACQUISITIONS
27.6 NEW PRODUCT DEVELOPMENT & APPROVALS
27.7 EXPANSIONS
27.8 REGULATORY CHANGES
27.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
28 GLOBAL TUMOR LYSIS SYNDROME MARKET, BY GEOGRAPHY
GLOBAL TUMOR LYSIS SYNDROME MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
28.1 NORTH AMERICA
28.1.1 U.S.
28.1.2 CANADA
28.1.3 MEXICO
28.2 EUROPE
28.2.1 GERMANY
28.2.2 U.K.
28.2.3 ITALY
28.2.4 FRANCE
28.2.5 SPAIN
28.2.6 RUSSIA
28.2.7 SWITZERLAND
28.2.8 TURKEY
28.2.9 BELGIUM
28.2.10 NETHERLANDS
28.2.11 DENMARK
28.2.12 SWEDEN
28.2.13 POLAND
28.2.14 NORWAY
28.2.15 FINLAND
28.2.16 REST OF EUROPE
28.3 ASIA-PACIFIC
28.3.1 JAPAN
28.3.2 CHINA
28.3.3 SOUTH KOREA
28.3.4 INDIA
28.3.5 SINGAPORE
28.3.6 THAILAND
28.3.7 INDONESIA
28.3.8 MALAYSIA
28.3.9 PHILIPPINES
28.3.10 AUSTRALIA
28.3.11 NEW ZEALAND
28.3.12 VIETNAM
28.3.13 TAIWAN
28.3.14 REST OF ASIA-PACIFIC
28.4 SOUTH AMERICA
28.4.1 BRAZIL
28.4.2 ARGENTINA
28.4.3 REST OF SOUTH AMERICA
28.5 MIDDLE EAST AND AFRICA
28.5.1 SOUTH AFRICA
28.5.2 EGYPT
28.5.3 BAHRAIN
28.5.4 UNITED ARAB EMIRATES
28.5.5 KUWAIT
28.5.6 OMAN
28.5.7 QATAR
28.5.8 SAUDI ARABIA
28.5.9 REST OF MIDDLE EAST AND AFRICA
28.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
29 GLOBAL TUMOR LYSIS SYNDROME MARKET, SWOT AND DBMR ANALYSIS
30 GLOBAL TUMOR LYSIS SYNDROME MARKET, COMPANY PROFILE
30.1 NORTHSTAR RX LLC.
30.1.1 COMPANY OVERVIEW
30.1.2 REVENUE ANALYSIS
30.1.3 GEOGRAPHIC PRESENCE
30.1.4 PRODUCT PORTFOLIO
30.1.5 RECENT DEVELOPMENTS
30.2 SUN PHARMACEUTICAL INDUSTRIES LTD.
30.2.1 COMPANY OVERVIEW
30.2.2 REVENUE ANALYSIS
30.2.3 GEOGRAPHIC PRESENCE
30.2.4 PRODUCT PORTFOLIO
30.2.5 RECENT DEVELOPMENTS
30.3 HARMAN FINOCHEM LTD
30.3.1 COMPANY OVERVIEW
30.3.2 REVENUE ANALYSIS
30.3.3 GEOGRAPHIC PRESENCE
30.3.4 PRODUCT PORTFOLIO
30.3.5 RECENT DEVELOPMENTS
30.4 VIATRIS INC.
30.4.1 COMPANY OVERVIEW
30.4.2 REVENUE ANALYSIS
30.4.3 GEOGRAPHIC PRESENCE
30.4.4 PRODUCT PORTFOLIO
30.4.5 RECENT DEVELOPMENTS
30.5 INDOCO REMEDIES LIMITED
30.5.1 COMPANY OVERVIEW
30.5.2 REVENUE ANALYSIS
30.5.3 GEOGRAPHIC PRESENCE
30.5.4 PRODUCT PORTFOLIO
30.5.5 RECENT DEVELOPMENTS
30.6 IPCA LABORATORIES LTD.
30.6.1 COMPANY OVERVIEW
30.6.2 REVENUE ANALYSIS
30.6.3 GEOGRAPHIC PRESENCE
30.6.4 PRODUCT PORTFOLIO
30.6.5 RECENT DEVELOPMENTS
30.7 PAR FORMULATIONS PRIVATE LIMITED
30.7.1 COMPANY OVERVIEW
30.7.2 REVENUE ANALYSIS
30.7.3 GEOGRAPHIC PRESENCE
30.7.4 PRODUCT PORTFOLIO
30.7.5 RECENT DEVELOPMENTS
30.8 CELON LABS
30.8.1 COMPANY OVERVIEW
30.8.2 REVENUE ANALYSIS
30.8.3 GEOGRAPHIC PRESENCE
30.8.4 PRODUCT PORTFOLIO
30.8.5 RECENT DEVELOPMENTS
30.9 LUPIN
30.9.1 COMPANY OVERVIEW
30.9.2 REVENUE ANALYSIS
30.9.3 GEOGRAPHIC PRESENCE
30.9.4 PRODUCT PORTFOLIO
30.9.5 RECENT DEVELOPMENTS
30.1 ACCORD HEALTHCARE (INTAS PHARMACEUTICALS)
30.10.1 COMPANY OVERVIEW
30.10.2 REVENUE ANALYSIS
30.10.3 GEOGRAPHIC PRESENCE
30.10.4 PRODUCT PORTFOLIO
30.10.5 RECENT DEVELOPMENTS
30.11 CHARTWELLPHARMA
30.11.1 COMPANY OVERVIEW
30.11.2 REVENUE ANALYSIS
30.11.3 GEOGRAPHIC PRESENCE
30.11.4 PRODUCT PORTFOLIO
30.11.5 RECENT DEVELOPMENTS
30.12 PATHEON MFG. SERVICES LLC
30.12.1 COMPANY OVERVIEW
30.12.2 REVENUE ANALYSIS
30.12.3 GEOGRAPHIC PRESENCE
30.12.4 PRODUCT PORTFOLIO
30.12.5 RECENT DEVELOPMENTS
30.13 SANOFI S.R.L.
30.13.1 COMPANY OVERVIEW
30.13.2 REVENUE ANALYSIS
30.13.3 GEOGRAPHIC PRESENCE
30.13.4 PRODUCT PORTFOLIO
30.13.5 RECENT DEVELOPMENTS
30.14 TAJ PHARMA GROUP
30.14.1 COMPANY OVERVIEW
30.14.2 REVENUE ANALYSIS
30.14.3 GEOGRAPHIC PRESENCE
30.14.4 PRODUCT PORTFOLIO
30.14.5 RECENT DEVELOPMENTS
30.15 AETOS PHARMA PRIVATE LIMITED
30.15.1 COMPANY OVERVIEW
30.15.2 REVENUE ANALYSIS
30.15.3 GEOGRAPHIC PRESENCE
30.15.4 PRODUCT PORTFOLIO
30.15.5 RECENT DEVELOPMENTS
30.16 ACTIZAPHARMA.COM
30.16.1 COMPANY OVERVIEW
30.16.2 REVENUE ANALYSIS
30.16.3 GEOGRAPHIC PRESENCE
30.16.4 PRODUCT PORTFOLIO
30.16.5 RECENT DEVELOPMENTS
30.17 G J PHARMACEUTICALS LLP
30.17.1 COMPANY OVERVIEW
30.17.2 REVENUE ANALYSIS
30.17.3 GEOGRAPHIC PRESENCE
30.17.4 PRODUCT PORTFOLIO
30.17.5 RECENT DEVELOPMENTS
30.18 UNICHEM PHARMACEUTICALS USA INC
30.18.1 COMPANY OVERVIEW
30.18.2 REVENUE ANALYSIS
30.18.3 GEOGRAPHIC PRESENCE
30.18.4 PRODUCT PORTFOLIO
30.18.5 RECENT DEVELOPMENTS
30.19 ZYDUS PHARMACEUTICALS, INC.
30.19.1 COMPANY OVERVIEW
30.19.2 REVENUE ANALYSIS
30.19.3 GEOGRAPHIC PRESENCE
30.19.4 PRODUCT PORTFOLIO
30.19.5 RECENT DEVELOPMENTS
30.2 DR. REDDY’S LABORATORIES, INC.
30.20.1 COMPANY OVERVIEW
30.20.2 REVENUE ANALYSIS
30.20.3 GEOGRAPHIC PRESENCE
30.20.4 PRODUCT PORTFOLIO
30.20.5 RECENT DEVELOPMENTS
30.21 WATSONS
30.21.1 TAKEDA COMPANY OVERVIEW
30.21.2 REVENUE ANALYSIS
30.21.3 GEOGRAPHIC PRESENCE
30.21.4 PRODUCT PORTFOLIO
30.21.5 RECENT DEVELOPMENTS
30.22 PHARMACEUTICAL COMPANY LIMITED
30.22.1 COMPANY OVERVIEW
30.22.2 REVENUE ANALYSIS
30.22.3 GEOGRAPHIC PRESENCE
30.22.4 PRODUCT PORTFOLIO
30.22.5 RECENT DEVELOPMENTS
30.23 AVET PHARMACEUTICALS INC
30.23.1 COMPANY OVERVIEW
30.23.2 REVENUE ANALYSIS
30.23.3 GEOGRAPHIC PRESENCE
30.23.4 PRODUCT PORTFOLIO
30.23.5 RECENT DEVELOPMENTS
30.24 ADVACARE PHARMA
30.24.1 COMPANY OVERVIEW
30.24.2 REVENUE ANALYSIS
30.24.3 GEOGRAPHIC PRESENCE
30.24.4 PRODUCT PORTFOLIO
30.24.5 RECENT DEVELOPMENTS
30.25 CAMBER PHARMACEUTICALS, INC.
30.25.1 COMPANY OVERVIEW
30.25.2 REVENUE ANALYSIS
30.25.3 GEOGRAPHIC PRESENCE
30.25.4 PRODUCT PORTFOLIO
30.25.5 RECENT DEVELOPMENTS
30.26 ALEMBIC PHARMACEUTICALS LIMITED
30.26.1 COMPANY OVERVIEW
30.26.2 REVENUE ANALYSIS
30.26.3 GEOGRAPHIC PRESENCE
30.26.4 PRODUCT PORTFOLIO
30.26.5 RECENT DEVELOPMENTS
30.27 MACLEODS PHARMA UK LIMITED
30.27.1 COMPANY OVERVIEW
30.27.2 REVENUE ANALYSIS
30.27.3 GEOGRAPHIC PRESENCE
30.27.4 PRODUCT PORTFOLIO
30.27.5 RECENT DEVELOPMENTS
30.28 LANNETT
30.28.1 COMPANY OVERVIEW
30.28.2 REVENUE ANALYSIS
30.28.3 GEOGRAPHIC PRESENCE
30.28.4 PRODUCT PORTFOLIO
30.28.5 RECENT DEVELOPMENTS
30.29 AUROBINDO PHARMA LIMITED
30.29.1 COMPANY OVERVIEW
30.29.2 REVENUE ANALYSIS
30.29.3 GEOGRAPHIC PRESENCE
30.29.4 PRODUCT PORTFOLIO
30.29.5 RECENT DEVELOPMENTS
30.3 NOVADOZ PHARMACEUTICALS
30.30.1 COMPANY OVERVIEW
30.30.2 REVENUE ANALYSIS
30.30.3 GEOGRAPHIC PRESENCE
30.30.4 PRODUCT PORTFOLIO
30.30.5 RECENT DEVELOPMENTS
30.31 WEST-WARD COLUMBUS INC.
30.31.1 COMPANY OVERVIEW
30.31.2 REVENUE ANALYSIS
30.31.3 GEOGRAPHIC PRESENCE
30.31.4 PRODUCT PORTFOLIO
30.31.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
31 RELATED REPORTS
32 CONCLUSION
33 QUESTIONNAIRE
34 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.