Global Twin Twin Transfusion Syndrome Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
752.40 Million
USD
1,069.98 Million
2025
2033
USD
752.40 Million
USD
1,069.98 Million
2025
2033
| 2026 –2033 | |
| USD 752.40 Million | |
| USD 1,069.98 Million | |
|
|
|
|
Twin-Twin Transfusion Syndrome Treatment Market Size
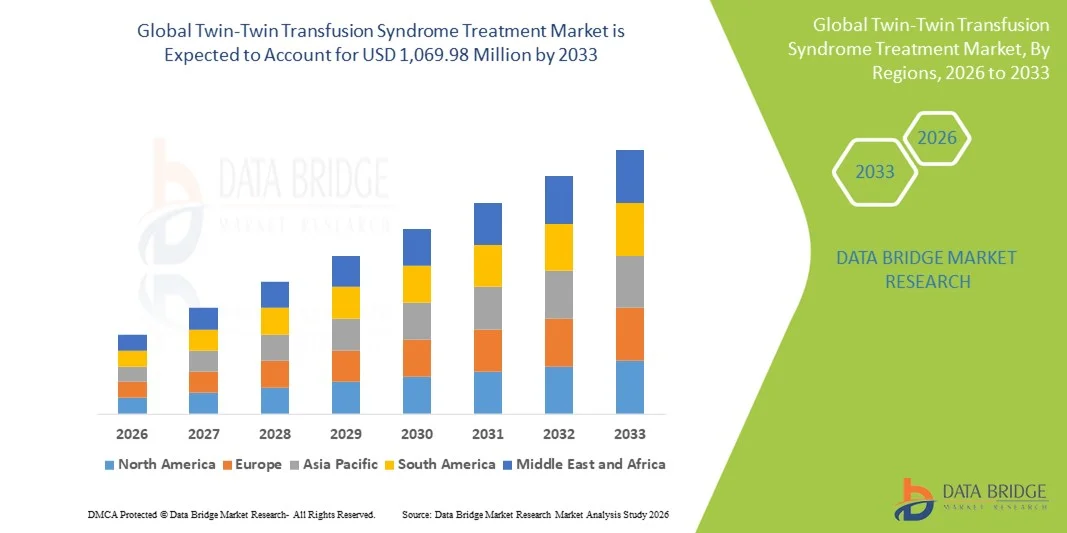
- The global Twin-Twin Transfusion Syndrome treatment market size was valued at USD 752.40 million in 2025 and is expected to reach USD 1,069.98 million by 2033, at a CAGR of 4.50% during the forecast period
- The market growth is primarily driven by rising awareness, improved diagnostic capabilities, and advancements in fetal therapy techniques, including fetoscopic laser photocoagulation (FLP), which remains the gold standard treatment for TTTS
- In addition, the increasing prevalence of monochorionic twin pregnancies, coupled with growing access to specialized maternal-fetal medicine centers, is significantly enhancing treatment adoption. These evolving clinical and technological developments are accelerating the uptake of TTTS treatment options, thereby strongly supporting market expansion
Twin-Twin Transfusion Syndrome Treatment Market Analysis
- Twin-Twin Transfusion Syndrome, a serious complication in monochorionic twin pregnancies, requires highly specialized fetal interventions, with fetoscopic laser photocoagulation emerging as the most effective treatment due to its ability to correct abnormal placental vascular connections and improve survival outcomes for both twins
- The rising demand for Twin-Twin Transfusion Syndrome treatment is primarily driven by increasing awareness of fetal therapy options, advancements in prenatal imaging, and the growing number of maternal-fetal medicine centers equipped to perform minimally invasive procedures
- North America dominated the global Twin-Twin Transfusion Syndrome treatment market with a revenue share of 41.2% in 2025, supported by advanced healthcare infrastructure, early adoption of fetal surgical technologies, and strong clinical expertise, with the U.S. experiencing a surge in fetoscopic procedures driven by expanding fetal care programs and continuous innovation in surgical visualization tools
- Asia-Pacific is expected to be the fastest-growing region, driven by rising birth rates, increasing access to high-level prenatal care, and rapid improvements in fetal therapy capabilities across emerging healthcare systems
- The surgery segment dominated the global Twin-Twin Transfusion Syndrome treatment market with a market share of 48.5% in 2025, driven by the widespread adoption of fetoscopic laser photocoagulation as the gold-standard intervention, its high success rate in addressing placental vascular imbalance, and the increasing global availability of specialized fetal surgery centers
Report Scope and Twin-Twin Transfusion Syndrome Treatment Market Segmentation
|
Attributes |
Twin-Twin Transfusion Syndrome Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Twin-Twin Transfusion Syndrome Treatment Market Trends
Advancement of Precision-Based Fetal Intervention Technologies
- A significant and accelerating trend in the global Twin-Twin Transfusion Syndrome treatment market is the rapid enhancement of precision-based fetal intervention tools, particularly improvements in fetoscopic laser systems that enable safer and more accurate coagulation of abnormal placental vessels, thereby improving survival outcomes for affected twins
- For instance, the use of high-definition fetoscopes with upgraded visualization capabilities allows maternal-fetal surgeons to identify vascular anastomoses with greater clarity, supporting more effective procedures and reducing postoperative complications
- Integrating digital imaging enhancements into fetoscopic platforms enables features such as improved vessel mapping, real-time surgical navigation, and more accurate targeting during laser ablation. For instance, several advanced fetal therapy centers are adopting imaging systems that provide clearer intrauterine visualization and enhanced detection of subtle vascular patterns essential for successful intervention
- The adoption of digitally enhanced fetal surgery platforms supports more streamlined coordination with prenatal imaging systems, allowing clinicians to synchronize ultrasound data, intraoperative visuals, and patient monitoring for more comprehensive fetal care
- This trend toward more advanced, precise, and integrated fetal surgery technologies is reshaping expectations for minimally invasive fetal therapy. Consequently, companies developing specialized surgical tools are focusing on improving illumination quality, visualization accuracy, and laser performance for enhanced clinical outcomes
- The demand for treatment options that incorporate improved imaging, refined surgical navigation, and advanced laser capabilities is increasing rapidly across major healthcare systems, as clinicians and parents prioritize safer and more effective intervention pathways
Twin-Twin Transfusion Syndrome Treatment Market Dynamics
Driver
Growing Adoption of Fetoscopic Procedures Driven by Rising Clinical Awareness and Advancing Fetal Care Infrastructure
- The increasing recognition of severe pregnancy complications associated with monochorionic twin gestations, coupled with expanding clinical awareness of effective fetal therapy interventions, is a major driver fueling the need for Twin-Twin Transfusion Syndrome treatment
- For instance, in recent years, multiple fetal therapy programs across major hospitals have expanded their fetoscopic capabilities, integrating advanced laser platforms and updated procedural protocols to enhance treatment outcomes, thereby strengthening overall market growth
- As more clinicians become aware of the importance of timely diagnosis and intervention, advanced fetal surgery options provide substantial benefits, including improved survival rates, reduced neurological risks, and enhanced long-term prognoses for twins affected by the condition
- Furthermore, the growing availability of advanced prenatal imaging systems and the rising focus on specialized maternal-fetal medicine units are making fetoscopic procedures a more accessible and standardized component of high-risk pregnancy management
- The clinical advantages offered by minimally invasive fetal interventions, including targeted vessel ablation and reduced procedural recovery times, are key factors accelerating adoption across developed and emerging regions. The expansion of fetal care networks and greater investments in specialized surgical training further reinforce this momentum
Restraint/Challenge
Limited Access to Specialized Fetal Surgery Centers and Procedural Complexity Hurdle
- The significant challenges persist due to the limited number of medical centers equipped to perform complex minimally invasive fetal surgeries required for Twin-Twin Transfusion Syndrome treatment, creating geographic disparities in access and delaying timely intervention for many patients
- For instance, the scarcity of highly trained maternal-fetal surgeons and the concentration of advanced fetal therapy units in major metropolitan regions make it difficult for patients in remote or underserved areas to receive the specialized care necessary for optimal outcomes
- Addressing these barriers through expanded training programs, increased funding for fetal surgery infrastructure, and broader integration of advanced prenatal diagnostic systems is essential for improving patient access. In addition, the technical complexity of fetoscopic laser photocoagulation requires substantial expertise, limiting widespread procedural availability across general hospitals
- While global efforts are underway to enhance clinical capabilities, the significant investment required for advanced imaging systems, surgical equipment, and specialized training continues to be a limiting factor, especially in developing regions where healthcare resources remain constrained
- Overcoming these challenges through expanded maternal-fetal medicine networks, improved referral systems, and greater governmental support will be critical for enabling broader adoption of these life-saving fetal interventions
Twin-Twin Transfusion Syndrome Treatment Market Scope
The market is segmented on the basis of symptoms, treatment, mode of administration, distribution channel, and end user.
- By Symptoms
On the basis of symptoms, the TTTS market is segmented into breathlessness, tightness in the abdomen, rapid expansion of abdomen, rapid weight gain, pressure on the stomach, and premature contractions. The rapid expansion of the abdomen segment dominated the market, driven by its strong clinical correlation with abnormal amniotic fluid accumulation in TTTS cases. This symptom is among the earliest and most visible indicators of fetal fluid imbalance, prompting urgent diagnostic ultrasound scans. Obstetricians rely heavily on this symptom to guide swift referrals to fetal medicine specialists, increasing treatment demand. Due to its high frequency among TTTS mothers, it drives consistent clinical visits and contributes significantly to the treatment volume. Early identification of abdominal enlargement helps prevent TTTS progression, strengthening its role in clinical decision-making. As awareness rises among pregnant women and clinicians, this segment continues to lead market share.
Premature contractions are anticipated to witness the fastest growth rate from 2026 to 2033, fueled by increased use of continuous maternal monitoring and digital pregnancy devices. These contractions often indicate acute uterine stress associated with TTTS, leading to rapid hospitalization and intervention. With heightened awareness among healthcare professionals, the recognition of contraction patterns has improved, resulting in earlier TTTS diagnosis. Telemedicine services now allow expectant mothers to report contraction symptoms more efficiently, enhancing screening volume. As premature contractions frequently trigger emergency evaluations, their link to immediate treatment drives market growth. Advances in prenatal care protocols are expected to accelerate this segment’s expansion.
- By Treatment
On the basis of treatment, the TTTS market is segmented into surgery, drugs, and others. The surgery segment dominated the market with the largest share of 48.5% in 2025, driven by the widespread clinical adoption of fetoscopic laser photocoagulation the gold standard treatment for TTTS. This procedure directly seals abnormal placental blood vessel connections, offering superior survival outcomes compared to supportive therapies. Increasing investment in fetal therapy centers and advanced surgical imaging technologies supports this dominance. Hospitals and maternal-fetal medicine units prioritize surgery because it addresses the root cause of TTTS rather than providing symptomatic relief. Clinical guidelines worldwide strongly recommend laser surgery for stages II–IV TTTS, reinforcing demand. As more healthcare systems adopt minimally invasive fetal procedures, the surgical segment maintains its leadership position.
The drugs segment is expected to grow at the fastest rate from 2026 to 2033, supported by rising adoption of pharmacological therapies such as tocolytics, corticosteroids, and anti-inflammatory agents. These medications are essential for stabilizing expectant mothers before and after laser surgery, increasing their use in TTTS care pathways. Regions with limited access to fetal surgery rely more heavily on drug-based management, boosting demand globally. Research efforts focusing on maternal-fetal targeted drugs are expanding, encouraging clinical adoption. Oral and injectable drug options provide flexibility for different stages of treatment. As healthcare providers move toward multidisciplinary TTTS management, pharmaceutical intervention is expected to rise rapidly.
- By Mode of Administration
On the basis of mode of administration, the market is segmented into injectable, oral, and others. The injectable segment dominated the market with the largest revenue share due to its critical role in delivering fast-acting therapies during TTTS emergencies and during surgical procedures. Many drugs used for maternal stabilization such as IV tocolytics and anesthesia require injectable administration for rapid clinical impact. Hospitals and fetal surgery centers prefer intravenous methods for precision dosing, especially during perioperative care. Injectable steroids used to prevent preterm birth also contribute to high usage in TTTS management. The segment benefits from consistent demand across all TTTS treatment stages, including diagnosis, preparation, and recovery. As surgical volumes increase globally, the injectable segment continues to hold the largest share.
The oral segment is anticipated to register the fastest CAGR from 2026 to 2033, driven by growing reliance on oral medications for prolonged maternal stabilization. These include oral tocolytics and other supportive therapies that help maintain pregnancy after TTTS treatment. The segment benefits from patient convenience and non-invasive delivery, contributing to improved adherence. As telemedicine expands, clinicians increasingly prescribe oral medications for at-home monitoring. Pharmaceutical advancements have resulted in safer and more effective formulations suitable for high-risk pregnancies. Broader distribution through retail and online pharmacies also accelerates this segment’s growth.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies dominated the market in 2025 due to the highly specialized nature of TTTS treatment, which requires hospital-based surgeries and immediate drug availability. TTTS management involves emergency stabilization, surgical intervention, and intensive post-treatment monitoring all of which rely on hospital pharmacy dispensing. Injectable medications and anesthesia used during fetal procedures are exclusively sourced from hospital pharmacies. Strong integration with maternal-fetal medicine units ensures seamless medication delivery and oversight. Increasing hospital admission rates for high-risk pregnancies further strengthen segment dominance. As more centers adopt advanced fetal surgery programs, the demand for hospital-based medication supply remains high.
Online pharmacies are expected to grow at the fastest rate from 2026 to 2033, driven by rising digitalization of healthcare and increased comfort with e-prescriptions. TTTS patients often require long-term oral medications after treatment, making online ordering highly convenient. Home delivery services benefit pregnant women who require reduced mobility, especially in high-risk conditions. Growing regulatory support for digital pharmacy platforms in major markets enhances consumer trust. Online portals offer better price transparency, prompting wider adoption among patients. As telehealth becomes integral to prenatal care, online pharmacies are expected to see significant expansion.
- By End User
On the basis of end user, the market is segmented into hospitals, homecare, specialty clinics, and others. The hospitals segment dominated the market with the largest share, supported by their central role in providing diagnosis, advanced imaging, and surgical treatment for TTTS. Most TTTS cases are identified during routine or emergency hospital scans, driving immediate admission for specialized care. Hospitals are equipped with fetal laser systems, NICUs, and multidisciplinary obstetric teams required for TTTS interventions. Their ability to manage high-risk pregnancies and preterm birth complications reinforces their importance. Growing investment in maternal-fetal medicine departments globally fuels segment dominance. As laser surgery adoption increases, hospitals remain the primary treatment centers.
Specialty clinics including dedicated fetal medicine and maternal-fetal therapy centers are projected to grow at the fastest rate during forecast period. Their expansion is driven by increasing demand for specialized expertise in diagnosing and treating complex fetal conditions such as TTTS. These clinics offer advanced ultrasound imaging and minimally invasive surgical capabilities, attracting referrals from general obstetric providers. Growing awareness of specialized fetal care has increased patient preference for these centers. Investments in standalone fetal therapy institutes in developed markets further boost growth. As healthcare systems encourage sub-specialization in obstetrics, the specialty clinics segment is poised for strong expansion.
Twin-Twin Transfusion Syndrome Treatment Market Regional Analysis
- North America dominated the global Twin-Twin Transfusion Syndrome treatment market with a revenue share of 41.2% in 2025, supported by advanced healthcare infrastructure, early adoption of fetal surgical technologies, and strong clinical expertise, with the U.S. experiencing a surge in fetoscopic procedures driven by expanding fetal care programs and continuous innovation in surgical visualization tools
- Expectant mothers in the region benefit from well-established prenatal screening programs, enabling the early detection of high-risk pregnancies, which significantly increases treatment uptake for TTTS
- The growing preference for minimally invasive fetal surgery, coupled with strong healthcare reimbursement frameworks and the availability of experienced fetal surgeons, further accelerates treatment adoption across hospitals and specialty clinics
U.S. Twin-Twin Transfusion Syndrome Treatment Market Insight
The U.S. TTTS treatment market captured the largest revenue share within North America in 2025, driven by the strong presence of advanced fetal therapy centers and widespread access to high-quality prenatal care. Increasing adoption of routine ultrasound screening and early diagnosis programs significantly improves detection rates, boosting treatment demand. The growing availability of fetoscopic laser photocoagulation in major U.S. hospitals further strengthens market growth. Moreover, rising awareness of high-risk pregnancies among expectant mothers, combined with robust insurance coverage for fetal procedures, supports rapid adoption. Continued advancements in fetal surgery techniques and maternal-fetal medicine are expected to enhance the country’s leadership in TTTS treatment.
Europe Twin-Twin Transfusion Syndrome Treatment Market Insight
The Europe TTTS treatment market is projected to expand at a substantial CAGR throughout the forecast period, largely fueled by strong healthcare infrastructure and early adoption of specialized fetal surgery techniques. Increasing focus on maternal and neonatal health standards across European countries supports widespread implementation of TTTS screening protocols. The region’s emphasis on timely diagnosis, along with the availability of skilled fetal surgeons, contributes to rising treatment volumes. Furthermore, growing awareness programs and improved access to high-resolution ultrasound technologies are enhancing early detection. The integration of advanced fetal therapy services into both public and private healthcare systems continues to support market growth across Europe.
U.K. Twin-Twin Transfusion Syndrome Treatment Market Insight
The U.K. TTTS treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by the strong presence of national fetal medicine units and widespread access to specialized treatment pathways. Rising awareness of prenatal complications and the high adoption of routine fetal scans drive increased TTTS detection. The U.K.’s strong focus on maternal safety and structured referral systems enhances timely access to fetal therapy. Advanced clinical research from leading institutions further strengthens treatment capabilities. In addition, government-backed healthcare services and strong public awareness programs continue to support expanded adoption of TTTS interventions.
Germany Twin-Twin Transfusion Syndrome Treatment Market Insight
The Germany TTTS treatment market is expected to expand at a considerable CAGR during the forecast period, driven by the country’s advanced healthcare infrastructure and strong emphasis on medical technology innovation. Widespread access to fetal medicine specialists and high-precision diagnostic tools contributes to early identification of TTTS cases. Germany’s leadership in minimally invasive surgical technologies further supports adoption of fetoscopic laser therapy. Rising public awareness of prenatal risks and increasing utilization of specialized obstetric centers strengthen market penetration. As the country continues to emphasize high standards of maternal care, demand for TTTS treatment is expected to grow consistently.
Asia-Pacific Twin-Twin Transfusion Syndrome Treatment Market Insight
The Asia-Pacific TTTS treatment market is poised to grow at the fastest CAGR from 2026 to 2033, driven by rapid improvements in prenatal care infrastructure and increasing access to ultrasound technologies in countries such as China, Japan, and India. Rising awareness of fetal complications and growing investment in maternal health programs are significantly improving TTTS diagnosis rates. The region’s expanding network of maternal-fetal medicine specialists supports greater adoption of advanced treatments. Furthermore, government initiatives promoting improved prenatal screening and high-risk pregnancy management are contributing to accelerated market growth. As APAC continues strengthening neonatal care systems, TTTS treatment adoption is expected to surge.
Japan Twin-Twin Transfusion Syndrome Treatment Market Insight
The Japan TTTS treatment market is gaining momentum due to the country’s strong focus on maternal-fetal healthcare, advanced medical technology landscape, and high awareness of prenatal screening. Japan’s well-established hospital systems support early TTTS detection through routine and high-frequency ultrasounds. The growing number of fetal therapy centers equipped with fetoscopic surgical technologies significantly enhances treatment capabilities. Integration of TTTS management into specialized perinatal care units further strengthens outcomes. Moreover, Japan’s aging maternal population is such likely to increase screening and monitoring needs, supporting continued market growth across both public and private healthcare sectors.
India Twin-Twin Transfusion Syndrome Treatment Market Insight
The India TTTS treatment market accounted for one of the significant shares in Asia-Pacific in 2025, driven by rapid improvements in prenatal screening infrastructure and increased access to maternal healthcare services. Rising awareness of high-risk pregnancies and growing utilization of ultrasound diagnostics support higher detection rates. The expansion of fetal medicine specialists and specialized perinatal care centers is improving availability of advanced TTTS treatments. Government initiatives focused on maternal and child health, along with growing uptake of private healthcare services, further strengthen market adoption. As India continues prioritizing neonatal and prenatal care, demand for TTTS interventions is expected to rise steadily.
Twin-Twin Transfusion Syndrome Treatment Market Share
The Twin-Twin Transfusion Syndrome Treatment industry is primarily led by well-established companies, including:
- KARL STORZ SE & Co. KG (Germany)
- Richard Wolf GmbH (Germany)
- Cook (U.S.)
- Olympus Corporation (Japan)
- Stryker (U.S.)
- Smith & Nephew (U.K.)
- B. Braun SE (Germany)
- Medtronic (Ireland)
- Boston Scientific Corporation (U.S.)
- Lumenis Be Ltd (Israel)
- Johnson & Johnson Services, Inc. (U.S.)
- Teleflex Incorporated (U.S.)
- CONMED Corporation (U.S.)
- FUJIFILM Holdings Corporation (Japan)
- PENTAX Medical America, Inc. (Japan)
- ERBE Elektromedizin GmbH (Germany)
- Getinge AB (Sweden)
- Storz Medical AG (Switzerland)
- Mauna Kea Technologies (France)
- Integra LifeSciences Corporation (U.S.)
What are the Recent Developments in Global Twin-Twin Transfusion Syndrome Treatment Market?
- In June 2025, a review article highlighted the growing role of angiogenic biomarkers in maternal blood post-laser therapy, suggesting these could predict fetal outcomes or risk of demise after surgery. The review emphasizes that changes in these soluble VEGF receptors may reflect placental vascular injury or repair after photocoagulation
- In March 2025, Baylor College of Medicine initiated a clinical trial (NCT06829901) to study how different uterine entry techniques (direct trocar vs. Seldinger method) during fetoscopic laser surgery influence the rate of chorioamniotic membrane separation.This trial is important because membrane separation is a known risk in fetoscopic procedures, and the entry method could impact surgical safety and outcomes
- In April 2024, a rare clinical case was published in Maternal-Fetal Medicine journal, reporting successful use of fetoscopic laser photocoagulation in dizygotic monochorionic (MCDZ) twins a very unusual but clinically significant presentation of TTTS. Monochorionicity is usually associated with monozygotic (identical) twins, but this case showed that dizygotic twins can also share a placenta and develop TTTS
- In January 2024, the Society for Fetal Medicine (SFM) issued updated clinical practice guidelines strongly recommending fetoscopic laser photocoagulation as the standard treatment for Quintero stage II–IV TTTS, based on contemporary outcome data. These guidelines reflect growing consensus in the medical community about early and aggressive intervention for more severe TTTS.
- In March 2021, Mayo Clinic highlighted that fetoscopic laser photocoagulation (FLP) especially when performed using the Solomon technique yields much better outcomes, with significantly higher dual-twin survival and lower recurrence of TTTS. This report underscored how surgical refinements in laser therapy over the years have boosted twin survival rates
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.