Global Viral Antigen Diagnostics Market
Market Size in USD Billion
CAGR :
% 
 USD
16.49 Billion
USD
30.30 Billion
2024
2032
USD
16.49 Billion
USD
30.30 Billion
2024
2032
| 2025 –2032 | |
| USD 16.49 Billion | |
| USD 30.30 Billion | |
|
|
|
|
Viral Antigen Diagnostics Market Size
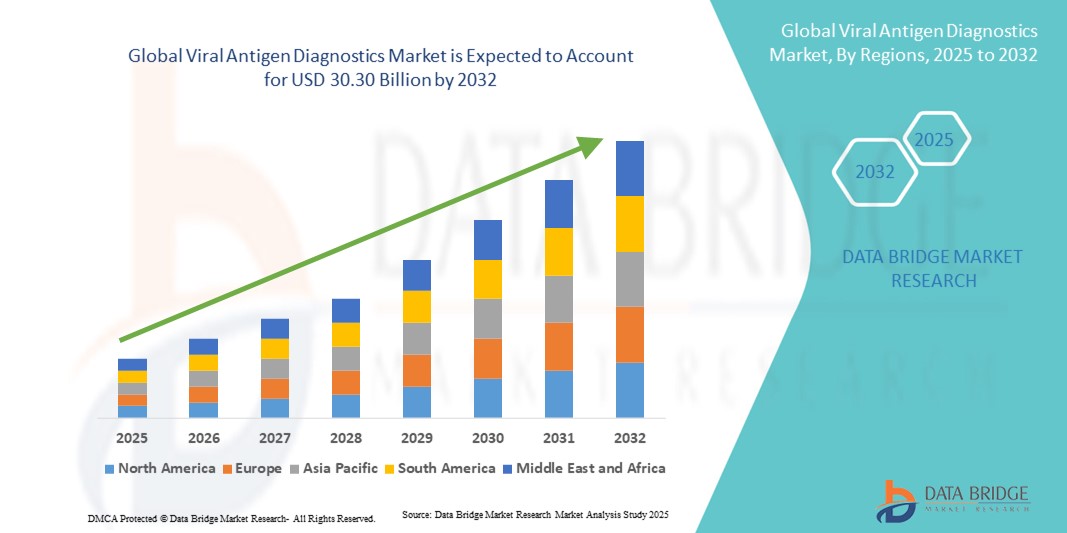
- The global viral antigen diagnostics market size was valued at USD 16.49 billion in 2024 and is expected to reach USD 30.30 billion by 2032, at a CAGR of 7.90% during the forecast period
- The market growth is largely fueled by the increasing prevalence of viral infections worldwide and the growing demand for rapid, accurate, and point-of-care diagnostic solutions across hospitals, clinics, and laboratories
- Furthermore, technological advancements in assay development, coupled with rising awareness of early detection and timely treatment of viral diseases, are driving the adoption of viral antigen diagnostics. These converging factors are accelerating the uptake of rapid diagnostic solutions, thereby significantly boosting the industry's growth
Viral Antigen Diagnostics Market Analysis
- Viral antigen diagnostics, offering rapid and specific detection of viral infections through antigen identification, are increasingly critical components of modern healthcare and point-of-care testing systems in both hospitals and laboratories due to their speed, accuracy, and ease of use
- The escalating demand for viral antigen diagnostics is primarily fueled by the rising prevalence of viral infections, growing awareness of early detection benefits, and a strong need for timely, reliable testing solutions across clinical and community settings
- North America dominated the viral antigen diagnostics market with the largest revenue share of 39.2% in 2024, characterized by advanced healthcare infrastructure, high healthcare expenditure, and a strong presence of leading diagnostic companies, with the U.S. experiencing substantial adoption of antigen testing, particularly in emergency care and community testing centers, driven by innovations in assay technology and digital reporting systems
- Asia-Pacific is expected to be the fastest-growing region in the viral antigen diagnostics market during the forecast period due to increasing healthcare investments, rising awareness of viral infections, and expanding diagnostic testing infrastructure in emerging economies
- Specimen Examination dominated the viral antigen diagnostics market with a share of 42.8% in 2024, driven by its ability to provide rapid and accurate results directly from patient samples, making it ideal for point-of-care testing and timely clinical decision-making
Report Scope and Viral Antigen Diagnostics Market Segmentation
|
Attributes |
Viral Antigen Diagnostics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Viral Antigen Diagnostics Market Trends
Advancements in Rapid and Point-of-Care Testing
- A significant and accelerating trend in the global viral antigen diagnostics market is the development of rapid, point-of-care testing platforms that deliver highly accurate results within minutes, enhancing early detection and disease management
- For instance, the BinaxNOW COVID-19 Ag Card enables healthcare providers to perform quick antigen testing at clinics and community testing centers with minimal equipment and training
- Integration of digital reporting and smartphone connectivity in diagnostic kits allows for real-time result sharing and epidemiological tracking, improving patient management and outbreak monitoring
- Advanced multiplex assays capable of detecting multiple viral antigens simultaneously are streamlining diagnostic workflows and reducing testing time, increasing efficiency in clinical settings
- This trend towards rapid, connected, and multiplex viral antigen tests is reshaping expectations for diagnostic speed and reliability, with companies such as Abbott developing portable antigen testing devices with digital reporting capabilities
- The demand for faster, user-friendly, and highly accurate viral antigen diagnostics is growing rapidly across hospitals, clinics, and public health programs, as stakeholders prioritize early detection and outbreak control
Viral Antigen Diagnostics Market Dynamics
Driver
Increasing Prevalence of Viral Infections and Early Detection Awareness
- The rising global prevalence of viral infections, coupled with growing awareness of the benefits of early detection, is a significant driver for the heightened demand for viral antigen diagnostics
- For instance, the introduction of the Sofia SARS Antigen FIA in 2024 highlighted rapid point-of-care detection in hospitals and outpatient settings, driving adoption
- As healthcare providers focus on timely diagnosis to reduce complications and transmission, viral antigen diagnostics offer speed and convenience compared to conventional molecular tests
- Public health initiatives and awareness campaigns are promoting routine viral testing, particularly in high-risk populations, thereby increasing market demand
- The ability to integrate testing solutions with digital health platforms for real-time monitoring and reporting further strengthens adoption in clinical and community healthcare settings
- Rising investments in healthcare infrastructure and government programs for infectious disease monitoring are expected to continue propelling the adoption of viral antigen diagnostics globally
Restraint/Challenge
Accuracy Concerns and Regulatory Compliance Hurdles
- Concerns regarding false positives or false negatives in viral antigen testing pose a significant challenge to broader market adoption, particularly in high-stakes clinical decision-making
- For instance, early reports of lower sensitivity in some influenza antigen tests caused hesitancy among clinicians in relying solely on antigen-based results
- Addressing these concerns requires stringent regulatory approvals, ongoing quality validation, and adherence to international diagnostic standards to ensure trust among healthcare providers
- In addition, variability in regulatory frameworks across regions can slow market entry for new diagnostic products, particularly in emerging economies with evolving healthcare policies
- Cost considerations for high-sensitivity antigen tests may also hinder adoption in resource-limited settings, even though rapid tests are generally more affordable than molecular alternatives
- Overcoming these challenges through enhanced assay sensitivity, rigorous validation, and alignment with regulatory standards will be vital for sustained growth in the viral antigen diagnostics market
Viral Antigen Diagnostics Market Scope
The market is segmented on the basis of diagnosis test, virus type, and end user.
- By Diagnosis Test
On the basis of diagnosis test, the viral antigen diagnostics market is segmented into specimen examination, serodiagnostic tests, and viral isolation. The specimen examination segment dominated the market with the largest market revenue share of 42.8% in 2024, driven by its ability to provide rapid, accurate results directly from patient samples, making it ideal for point-of-care testing. Hospitals and clinics often prefer specimen examination for its speed, reliability, and minimal equipment requirements. The segment also benefits from integration with digital reporting systems, improving patient management and epidemiological tracking. In addition, specimen examination methods are versatile, compatible with multiple viral antigens, and suitable for high-throughput diagnostic workflows.
The serodiagnostic tests segment is anticipated to witness the fastest growth rate of 24.1% from 2025 to 2032, fueled by increasing adoption in early infection screening and large-scale epidemiological studies. Serodiagnostic tests are highly useful for detecting immune responses to viral infections and provide supplementary information for disease monitoring. Growing awareness of their role in identifying prior exposure and guiding vaccination strategies further drives market expansion. Rising research initiatives and advancements in enzyme-linked and lateral flow serodiagnostic assays also contribute to this segment’s rapid growth.
- By Virus Type
On the basis of virus type, the viral antigen diagnostics market is segmented into Adenovirus, Cytomegalovirus, Dengue Virus, Enterovirus, Hepatitis Virus, HIV-1, Coronavirus, Human Metapneumovirus, Human Rhinovirus A, Measles Virus, Poliovirus, Rabies Virus, Varicella Zoster Virus, Avian Influenza, Coxsackievirus, Epstein-Barr Virus (EBV), Lymphocryptovirus, Herpes Virus (HSV-1, HSV-2), HIV-2, Human Herpesvirus, Human Papillomavirus (HPV), Influenza Virus, Mumps Virus, Rubella Virus, Respiratory Syncytial Virus (RSV), and West Nile Virus. The Coronavirus segment dominated the market with a share of 29.8% in 2024, driven by the ongoing global focus on COVID-19 detection and surveillance programs. Widespread adoption of rapid antigen tests for coronavirus in hospitals, clinics, and community testing centers contributed significantly to this share. Government-led screening programs, point-of-care testing initiatives, and high public awareness have reinforced its market dominance. Technological innovations improving sensitivity and specificity of coronavirus antigen tests further strengthen this segment.
The Dengue Virus segment is expected to witness the fastest CAGR from 2025 to 2032, fueled by increasing prevalence in tropical and subtropical regions and rising public health initiatives. Rapid detection of dengue antigens allows timely intervention and outbreak control. Investments in affordable, portable dengue diagnostic kits and expansion of testing infrastructure in emerging economies are accelerating adoption. Enhanced multiplex testing platforms that simultaneously detect dengue alongside other viral infections also contribute to the segment’s high growth potential.
- By End User
On the basis of end user, the viral antigen diagnostics market is segmented into physician offices, commercial laboratories, and nursing homes. The commercial laboratories segment held the largest market revenue share of 45.5% in 2024, attributed to their capacity to perform high-volume testing, access to advanced diagnostic technologies, and partnerships with healthcare providers for large-scale viral testing programs. Commercial laboratories also benefit from economies of scale, high operational efficiency, and the ability to offer rapid turnaround times, making them a preferred choice for hospitals and public health agencies.
The physician offices segment is expected to witness the fastest growth rate of 22.8% from 2025 to 2032, driven by the increasing adoption of point-of-care testing in outpatient settings. Easy-to-use antigen testing kits that provide immediate results enhance patient management and reduce the need for laboratory visits. Growing awareness among physicians and patients regarding early detection, combined with advancements in compact and user-friendly testing devices, is further accelerating adoption in this segment.
Viral Antigen Diagnostics Market Regional Analysis
- North America dominated the viral antigen diagnostics market with the largest revenue share of 39.2% in 2024, characterized by advanced healthcare infrastructure, high healthcare expenditure, and a strong presence of leading diagnostic companies
- Healthcare providers and public health agencies in the region prioritize rapid and accurate testing, valuing the convenience, speed, and reliability offered by viral antigen diagnostics for timely patient management and outbreak control
- This widespread adoption is further supported by high healthcare expenditure, robust regulatory frameworks, and ongoing investments in diagnostic technology, establishing viral antigen diagnostics as a preferred solution across hospitals, clinics, and community testing centers
U.S. Viral Antigen Diagnostics Market Insight
The U.S. viral antigen diagnostics market captured the largest revenue share of 82% in 2024 within North America, fueled by the widespread adoption of rapid and point-of-care testing. Healthcare providers and public health programs are increasingly prioritizing early detection of viral infections to reduce transmission and improve patient outcomes. The growing demand for portable and easy-to-use diagnostic kits, combined with integration with digital reporting and hospital information systems, further propels the market. Moreover, ongoing innovations in assay sensitivity and multiplex testing platforms are significantly contributing to market expansion.
Europe Viral Antigen Diagnostics Market Insight
The Europe viral antigen diagnostics market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing government initiatives for infectious disease control and widespread awareness of early diagnosis benefits. Rising urbanization and the growing prevalence of viral infections are fostering adoption across hospitals, clinics, and commercial laboratories. European healthcare providers are also attracted to the convenience, rapid turnaround, and high accuracy of antigen testing. The region is experiencing growth across diagnostic applications, including routine screening, outbreak management, and clinical surveillance.
U.K. Viral Antigen Diagnostics Market Insight
The U.K. viral antigen diagnostics market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by heightened demand for efficient and timely viral testing in both clinical and community settings. Increasing concerns regarding outbreaks and infection control are encouraging adoption among hospitals, physician offices, and public health agencies. The country’s advanced healthcare infrastructure and strong emphasis on early detection support market growth. Integration with electronic health records and point-of-care testing initiatives is expected to further stimulate adoption.
Germany Viral Antigen Diagnostics Market Insight
The Germany viral antigen diagnostics market is expected to expand at a considerable CAGR during the forecast period, fueled by awareness regarding early detection and preventive healthcare. Germany’s robust healthcare system, high-quality diagnostic laboratories, and government support for infectious disease monitoring promote adoption of viral antigen tests. Integration of rapid antigen diagnostics with hospital workflows and public health reporting systems is becoming increasingly prevalent. Demand for precise, high-sensitivity diagnostic solutions aligned with local regulatory standards further drives market growth.
Asia-Pacific Viral Antigen Diagnostics Market Insight
The Asia-Pacific viral antigen diagnostics market is poised to grow at the fastest CAGR of 25% during the forecast period of 2025 to 2032, driven by increasing healthcare investments, rising prevalence of viral infections, and expanding testing infrastructure in countries such as China, Japan, and India. The region’s growing focus on early detection and government initiatives promoting point-of-care testing are driving adoption. Furthermore, APAC’s emerging manufacturing capabilities and availability of affordable antigen testing kits are expanding access to a wider population.
Japan Viral Antigen Diagnostics Market Insight
The Japan viral antigen diagnostics market is gaining momentum due to the country’s advanced healthcare infrastructure, high public health awareness, and emphasis on rapid diagnostic solutions. Adoption is driven by the increasing number of clinics and hospitals offering point-of-care testing, as well as integration with electronic health records and connected diagnostic platforms. Japan’s aging population further increases demand for convenient, accurate, and timely viral antigen tests in both residential and clinical settings.
India Viral Antigen Diagnostics Market Insight
The India viral antigen diagnostics market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to growing healthcare access, increasing prevalence of viral infections, and expanding diagnostic testing infrastructure. India represents one of the largest markets for affordable point-of-care antigen tests, with growing adoption in hospitals, commercial laboratories, and community health programs. Government initiatives to strengthen infectious disease surveillance and the presence of local manufacturers offering cost-effective solutions are key factors propelling the market in India.
Viral Antigen Diagnostics Market Share
The viral antigen diagnostics industry is primarily led by well-established companies, including:
- Creative Diagnostics (U.S.)
- EUROIMMUN Medizinische Labordiagnostika AG (Germany)
- Alpha Diagnostic Intl. Inc. (U.S.)
- The Native Antigen Company (U.K.)
- BioFire Diagnostics (U.S.)
- Premier Medical (India)
- Innovative Diagnostics (France)
- Meridian Bioscience, Inc. (U.S.)
- VedaLab (France)
- DiaSorin S.p.A. (Italy)
- Hotgen Biotech (China)
- Immudex (Denmark)
- Revvity, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Abbott (U.S.)
- Siemens Healthineers AG (Germany)
- BD (U.S.)
- QuidelOrtho Corporation (U.S.)
- GenMark Diagnostics (U.S.)
What are the Recent Developments in Global Viral Antigen Diagnostics Market?
- In July 2025, Quest Diagnostics launched a new diagnostic laboratory test for the Oropouche virus, utilizing polymerase chain reaction (PCR) technology to detect viral RNA during early infection stages. Serology testing for antibody detection was also planned to follow later in the quarter, expanding the diagnostic capabilities for this emerging infectious disease
- In July 2025, Researchers from the Indian Institute of Science Education and Research (IISER), Pune, developed a rapid, low-cost diagnostic test capable of detecting both COVID-19 and Zika virus within 20 minutes without the need for specialized laboratory settings. Utilizing a modified synthetic RNA sequence known as a 'toehold switch,' the test produces a visible color change on a small paper disc, offering a promising solution for regions with limited healthcare infrastructure
- In May 2025, SEKISUI Diagnostics released the OSOM RSV Test, a rapid immunochromatographic assay designed for the qualitative detection of respiratory syncytial virus (RSV) nucleoprotein antigen in anterior nasal swab specimens. Providing results in just 15 minutes, this CLIA-waived test is suitable for point-of-care settings, facilitating timely diagnosis and treatment of RSV infections
- In July 2023, Roche Diagnostics India introduced the Elecsys HCV Duo, the country's first fully automated immunoassay capable of simultaneously detecting hepatitis C virus (HCV) antigens and antibodies from a single plasma or serum sample. This dual detection approach enables early-stage infection identification and monitoring of chronic cases, enhancing diagnostic efficiency and patient management
- In May 2023, The U.S. Food and Drug Administration (FDA) granted marketing authorization for the VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Reagent Pack and the Anti-SARS-CoV-2 Total Reagent Pack, both manufactured by Ortho-Clinical Diagnostics, Inc. These tests are designed for use with the VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Calibrator, facilitating the detection of SARS-CoV-2 antibodies and supporting the assessment of immune response in individuals
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.