Global Whim Syndrome Management Market
Market Size in USD Million
CAGR :
% 
 USD
7.56 Million
USD
19.03 Million
2024
2032
USD
7.56 Million
USD
19.03 Million
2024
2032
| 2025 –2032 | |
| USD 7.56 Million | |
| USD 19.03 Million | |
|
|
|
Whim Syndrome Management Market Analysis
The Whim Syndrome management market is driven by the growing awareness of the condition and advancements in treatment options. As rare diseases continue to gain more attention in the medical field, there has been a steady rise in the development of targeted therapies, including gene therapies and monoclonal antibodies, aimed at addressing the root causes of Whim Syndrome. Research investments in genetic disorders are fueling the market growth, as pharmaceutical companies explore novel approaches to treatment.
Healthcare providers and researchers are increasingly focusing on personalized medicine, which tailors interventions to the specific genetic makeup of patients. This trend is expected to enhance the efficacy of treatments and improve patient outcomes. In addition, with the increased number of clinical trials and scientific discoveries, the market is witnessing the introduction of new, more effective therapies, increasing the demand for better management solutions.
The market is also being shaped by advancements in diagnostic technologies, which allow for earlier and more accurate identification of Whim Syndrome, thereby facilitating timely intervention. As healthcare systems evolve and become more equipped to deal with rare genetic disorders, the Whim Syndrome management market is set to experience significant growth, spurred by a combination of scientific innovation and heightened awareness.
Whim Syndrome Management Market Size
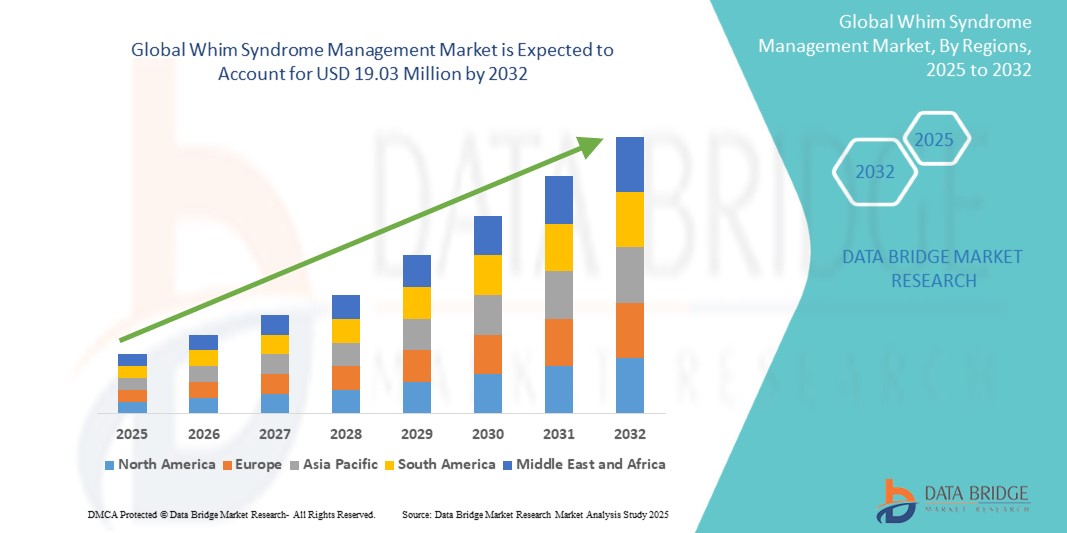
The global Whim Syndrome management market size was valued at USD 7.56 million in 2024 and is projected to reach USD 19.03 million by 2032, with a CAGR of 12.23% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Whim Syndrome Management Market Trends
“Growing Focus on Gene Therapy and Personalized Medicine”
A key trend in the Whim Syndrome Management market is the growing focus on gene therapy and personalized medicine. With the genetic basis of Whim Syndrome well-understood, there is a significant push toward developing targeted therapies that address the underlying mutations in the CXCR4 gene. Gene therapy, in particular, holds promise for offering long-term or even curative treatment options, reducing the reliance on traditional symptom management. Personalized medicine is also gaining traction, as treatments are increasingly tailored to the individual genetic profiles of patients, enhancing their efficacy and minimizing side effects. This trend is fueled by advancements in genetic research, biotechnology, and ongoing clinical trials aimed at better understanding the disorder’s pathophysiology. As these therapies become more refined, they could revolutionize the management of Whim Syndrome, improving patient outcomes and quality of life, while driving growth in the market.
Report Scope and Whim Syndrome Management Market Segmentation
|
Attributes |
Whim Syndrome Management Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
Norgine (The Netherlands), Sanofi (France) and X4 Pharmaceuticals, Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Whim Syndrome Management Market Definition
Whim Syndrome is a rare primary immunodeficiency disorder caused by mutations in the CXCR4 gene, which plays a critical role in the immune system. This condition is characterized by four key features: warts, hypogammaglobulinemia (low levels of immunoglobulins in the blood), infections, and myelokathexis (abnormal retention of neutrophils in the bone marrow). These abnormalities lead to a weakened immune system, making individuals more susceptible to recurrent infections and other complications. The management of Whim Syndrome focuses on addressing the underlying genetic mutations and controlling symptoms, often involving therapies that aim to increase the number of functional immune cells, such as neutrophils and lymphocytes. Treatments may include medications such as mavorixafor, a CXCR4 antagonist, which is designed to improve immune cell circulation and boost the immune system’s function.
Whim Syndrome Management Market Dynamics
Drivers
- Advancements in Targeted Therapies
One of the major drivers of the Whim Syndrome management market is the progress in targeted therapies, particularly CXCR4 antagonists such as mavorixafor. This selective CXCR4 receptor antagonist is designed to address the underlying cause of Whim Syndrome by improving the circulation of mature neutrophils and lymphocytes. For instance, X4 Pharmaceuticals' mavorixafor has shown significant promise in clinical trials and is now approved for use in the U.S. This advancement represents a major step forward in treating a rare and often misdiagnosed condition. With targeted therapies offering the potential for long-term improvements in immune function, patients are experiencing better health outcomes and fewer infections. The introduction of mavorixafor has not only paved the way for more effective treatments but has also increased the demand for specialized management solutions, driving growth in the Whim Syndrome management market.
- Growing Focus on Rare Diseases and Genetic Research
The increasing attention on rare diseases and genetic research is another key driver in the Whim Syndrome management market. Over the years, there has been an upswing in scientific understanding of genetic disorders, leading to the development of precision medicine tailored to individual patient needs. For instance, genetic testing and early diagnosis are now more accessible, enabling healthcare providers to intervene earlier and manage WHIM syndrome more effectively. In addition, the collaboration between pharmaceutical companies and research institutions has led to innovative therapies. The rising focus on genetic-based treatments, such as gene therapy and monoclonal antibodies, has resulted in more efficient and personalized treatment options. This trend has not only improved patient outcomes but also contributed to a growing interest in rare disease markets, expanding opportunities for market players and fueling the overall market growth.
Opportunities
- Expansion into Emerging Markets
A significant opportunity for growth in the Whim Syndrome management market lies in expanding access to treatments in emerging markets, particularly in regions with a rising focus on healthcare advancements. For instance, X4 Pharmaceuticals has entered into licensing agreements with companies such as Norgine and Taiba Rare to distribute XOLREMDI (mavorixafor) in regions such as Europe, the Middle East, and Australia. These regions, which may have previously lacked access to cutting-edge treatments, present substantial untapped potential. As healthcare infrastructure improves in emerging markets, demand for specialized treatments such as mavorixafor is expected to grow. By expanding into these regions, pharmaceutical companies can reach a broader patient base, accelerating both market penetration and revenue growth. The increasing availability of effective treatments in these areas is poised to drive significant market expansion and contribute to the overall growth of the Whim Syndrome management sector.
- Growing Research and Development in Gene Therapy
Another opportunity in the Whim Syndrome management market is the growing investment in gene therapy and other genetic-based treatments. Companies and research institutions are increasingly focusing on genetic disorders, aiming to develop personalized therapies that target the root causes of conditions such as Whim Syndrome. For instance, mavorixafor, a selective CXCR4 receptor antagonist, is part of the larger trend of advancing gene-targeted treatments for rare diseases. Ongoing research into gene therapies and CRISPR technology could open new avenues for curing or significantly improving outcomes for patients with genetic conditions. As these treatments become more refined and accessible, they are expected to further revolutionize the management of Whim Syndrome. The potential for breakthrough therapies will likely drive innovation in the market and significantly increase the demand for cutting-edge treatment options, supporting sustained growth in the Whim Syndrome management market.
Restraints/Challenges
- Regulatory and Approval Delays
A significant restraint in the Whim Syndrome management market is the regulatory and approval delays associated with new treatments. Due to the rare nature of Whim Syndrome, treatments such as mavorixafor (XOLREMDI) must undergo extensive clinical trials and regulatory reviews before reaching patients. The process of obtaining regulatory approvals from agencies such as the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) can be lengthy and complex, with potential delays in the approval of new therapies. For instance, even after successful trials, the approval process may extend over several years, limiting patient access to new treatments. These delays can restrict the timely introduction of new therapies into the market, slowing down the pace of innovation and hindering market growth. The uncertainty surrounding approval timelines can also deter investment in research and development, further stifling progress in the management of Whim Syndrome.
- Limited Awareness and Diagnosis
One of the key challenges in the Whim Syndrome management market is the limited awareness and diagnostic challenges surrounding the condition. Since Whim Syndrome is a rare genetic disorder, it is often misdiagnosed or not diagnosed until late in a patient’s life. Symptoms such as recurrent infections and warts are common to various other conditions, leading to delays in proper diagnosis. For instance, a misdiagnosis can lead to ineffective treatments, worsening patient outcomes, and increased healthcare costs. As awareness of rare diseases grows, diagnostic technologies and genetic testing are improving, but many healthcare providers, especially in underserved regions, may still lack the expertise to identify the condition early. This diagnostic challenge delays access to appropriate treatment and impedes market growth, as the potential patient population remains underserved until diagnosis is made.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Whim Syndrome Management Market Scope
The market is segmented on the basis of treatment type, drug class, patient type, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Treatment Type
- Medication
- Therapy
- Counseling
- Lifestyle Modification
Drug Class
- Mavorixafor
- Plerixafor
Patient Type
- Adults
- Children
- Geriatric
Distribution Channel
- Hospitals
- Clinics
- Online Pharmacies
- Retail Pharmacies
Whim Syndrome Management Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, treatment type, drug class, patient type, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the Whim Syndrome management market due to the region's advanced healthcare infrastructure, high levels of investment in rare disease research, and early adoption of innovative therapies. The U.S., in particular, has seen the approval of treatments such as mavorixafor (XOLREMDI), which is already being utilized for managing Whim Syndrome. In addition, the presence of major pharmaceutical companies and active clinical trials further bolsters North America's position as the leading market for Whim Syndrome treatments.
Asia-Pacific region is expected to exhibit the highest growth rate in the Whim Syndrome management market. This growth is driven by improving healthcare infrastructure, rising awareness of rare diseases, and increasing investment in biotechnology and pharmaceuticals. Countries such as China and India are focusing on expanding access to advanced treatments, including genetic therapies. In addition, with a large patient population and growing healthcare needs, the APAC region offers significant opportunities for market expansion and adoption of innovative therapies.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Whim Syndrome Management Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Whim Syndrome Management Market Leaders Operating in the Market Are:
- Norgine (The Netherlands)
- Sanofi (France)
- X4 Pharmaceuticals, Inc. (U.S.)
Latest Developments in Whim Syndrome Management Market
- In February 2025, X4 Pharmaceuticals and Taiba Rare announced an exclusive agreement for the distribution and commercialization of XOLREMDI (mavorixafor), an oral, once-daily treatment for WHIM syndrome (warts, hypogammaglobulinemia, infections, and myelokathexis), in select Middle Eastern countries, including Saudi Arabia, the United Arab Emirates, Qatar, Oman, Kuwait, Bahrain, and Egypt, pending regulatory approvals in the region.
- In January 2025, X4 Pharmaceuticals announced that the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) has validated its Marketing Authorization Application (MAA) for mavorixafor, a treatment for WHIM syndrome (warts, hypogammaglobulinemia, infections, and myelokathexis), a rare primary immunodeficiency. The application is now under evaluation.
- In January 2025, X4 Pharmaceuticals and Norgine announced an exclusive licensing and supply agreement, under which Norgine will commercialize mavorixafor in Europe, Australia, and New Zealand following regulatory approvals. Mavorixafor, a selective CXCR4 receptor antagonist, is approved in the U.S. and marketed by X4 Pharmaceuticals under the brand name XOLREMDI for patients with WHIM syndrome.
- In April 2024, X4 Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) has approved XOLREMDI (mavorixafor) capsules for patients aged 12 and older with WHIM syndrome (warts, hypogammaglobulinemia, infections, and myelokathexis) to help increase the number of circulating mature neutrophils and lymphocytes. XOLREMDI, a selective CXC chemokine receptor 4 (CXCR4) antagonist, is the first treatment specifically approved for WHIM syndrome patients.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.