Middle East Africa Multiplex Assays Market
Market Size in USD Million
CAGR :
% 
 USD
270.50 Million
USD
447.67 Million
2024
2032
USD
270.50 Million
USD
447.67 Million
2024
2032
| 2025 –2032 | |
| USD 270.50 Million | |
| USD 447.67 Million | |
|
|
|
|
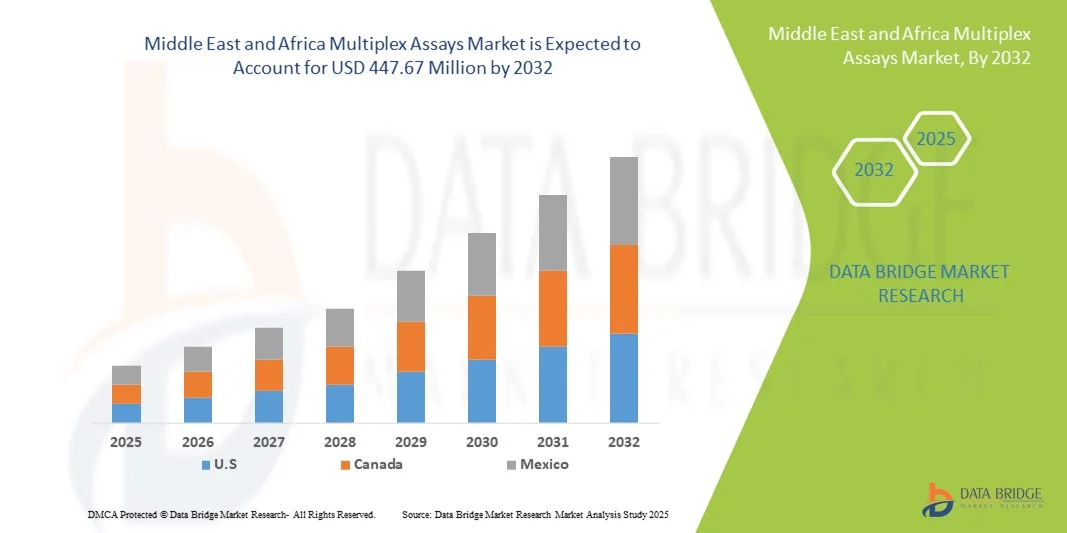
Middle East and Africa Multiplex Assays Market Size
- The Middle East and Africa multiplex assays market size was valued at USD 270.50 million in 2024 and is expected to reach USD 447.67 million by 2032, at a CAGR of 6.5% during the forecast period
- The market growth is largely fueled by increased research and development activities in the pharmaceutical sector, the adoption of multiplex tests for cancer and infectious disease diagnostics, and the advantages of multiplex assays over single-plex tests, driving higher efficiency in clinical and research applications
- Furthermore, rising investments in healthcare infrastructure, growing prevalence of chronic diseases, and technological advancements in assay techniques are establishing multiplex assays as essential tools for diagnostics and research in the region. These converging factors are accelerating the uptake of multiplex assay solutions, thereby significantly boosting the industry's growth
Middle East and Africa Multiplex Assays Market Analysis
- Multiplex assays, enabling simultaneous detection of multiple analytes in a single sample, are increasingly essential tools in clinical diagnostics, pharmaceutical research, and biomarker studies across both healthcare and research settings due to their high efficiency, reduced sample volume requirements, and cost-effectiveness
- The growing demand for multiplex assays is primarily driven by rising prevalence of chronic and infectious diseases, increased adoption of advanced diagnostic technologies, and the need for faster and more accurate testing in research and clinical laboratories
- South Africa dominated the Middle East and Africa multiplex assays market with the largest revenue share of 38.9% in 2024, characterized by significant healthcare infrastructure investments, strong government initiatives for advanced diagnostics, and presence of leading market players, with the country experiencing substantial growth in laboratory installations and diagnostic testing capabilities
- Saudi Arabia is expected to be the fastest growing country in the MEA multiplex assays market during the forecast period due to increasing healthcare expenditure, growing awareness about early disease detection, and rising adoption of innovative diagnostic solutions
- Reagents and consumables segment dominated the MEA multiplex assays market with a market share of 42.3% in 2024, driven by their essential role in assay execution, high repeat purchase rate, and widespread use across clinical diagnostics and research applications
Report Scope and Middle East and Africa Multiplex Assays Market Segmentation
|
Attributes |
Middle East and Africa Multiplex Assays Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Middle East and Africa
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Middle East and Africa Multiplex Assays Market Trends
Advancement Through High-Throughput and Automated Platforms
- A significant and accelerating trend in the MEA multiplex assays market is the adoption of high-throughput and automated assay platforms that enable faster, more reliable, and reproducible results across clinical diagnostics and research laboratories. This technological shift is significantly improving lab efficiency and throughput
- For instance, Luminex MAGPIX system integrates automated sample handling with multiplex detection, allowing simultaneous analysis of multiple biomarkers in a single run, reducing manual intervention and error. Similarly, Bio-Rad Bio-Plex 200 system offers automated multiplexing for research and clinical applications, enhancing operational efficiency
- Automation in multiplex assays allows laboratories to handle larger sample volumes, standardize workflows, and reduce turnaround times, while integrated software provides intelligent data analysis and quality control alerts. For instance, Thermo Fisher’s ProcartaPlex assays use automated readers and software to streamline cytokine profiling with minimal manual handling
- The integration of automated multiplex assay platforms with laboratory information management systems (LIMS) facilitates centralized control over sample tracking, assay setup, and result management, improving accuracy and compliance across laboratories
- This trend towards faster, more reliable, and automated multiplex assays is reshaping expectations for laboratory efficiency, prompting companies such as QIAGEN to develop high-throughput kits with integrated automation features for both clinical and research applications
- The demand for multiplex assays with high-throughput and automation capabilities is growing rapidly across hospitals, clinical labs, and research institutes, as labs increasingly prioritize efficiency, accuracy, and workflow standardization
Middle East and Africa Multiplex Assays Market Dynamics
Driver
Rising Demand Due to Growing Prevalence of Chronic and Infectious Diseases
- The increasing prevalence of chronic diseases and infectious conditions across the MEA region, coupled with growing awareness of early disease detection, is a significant driver for the heightened demand for multiplex assays
- For instance, in March 2024, South Africa’s National Health Laboratory Services expanded multiplex testing for tuberculosis and HIV co-infections, demonstrating the critical role of multiplex assays in disease management. Such initiatives by key healthcare providers are expected to drive the market growth in the forecast period
- As healthcare providers focus on rapid and accurate diagnostics, multiplex assays offer simultaneous detection of multiple biomarkers, reducing testing time and improving diagnostic efficiency compared to traditional single-analyte assays
- Furthermore, the adoption of multiplex assays is being accelerated by government healthcare programs and private laboratory expansions aimed at improving clinical diagnostics and biomarker-based research across the region
- The ability to monitor multiple disease markers in a single test, combined with growing investments in diagnostic infrastructure and laboratory modernization, is propelling the adoption of multiplex assays across hospitals, clinical laboratories, and research institutions
Restraint/Challenge
High Cost and Regulatory Compliance Constraints
- The relatively high cost of advanced multiplex assay kits and instruments, along with stringent regulatory requirements, poses a significant challenge to broader market penetration in the MEA region. As multiplex assays require specialized reagents, instruments, and trained personnel, cost barriers can limit adoption, especially in resource-constrained settings
- For instance, delays in obtaining regulatory approvals for new multiplex diagnostic kits in countries such as Saudi Arabia and UAE have made some laboratories hesitant to adopt innovative solutions, slowing market growth
- Addressing these cost and compliance challenges through subsidies, training programs, and simplified regulatory pathways is crucial for wider adoption. Companies such as Illumina and Randox emphasize providing comprehensive technical support and compliance documentation to facilitate regulatory approvals
- In addition, the high initial investment for automated multiplex systems compared to traditional assays can restrict usage in smaller laboratories or research institutes, limiting market expansion in certain regions
- While costs are gradually decreasing and affordable options are emerging, the perceived premium for multiplex assay technologies can still hinder widespread adoption, particularly in developing MEA countries
- Overcoming these hurdles through cost-effective solutions and streamlined regulatory processes will be vital for sustained market growth
Middle East and Africa Multiplex Assays Market Scope
The market is segmented on the basis of products & services, type, technology, application, and end user.
- By Products & Services
On the basis of products and services, the MEA multiplex assays market is segmented into reagents & consumables, instruments & accessories, and software & services. The reagents & consumables segment dominated the market with the largest revenue share of 42.3% in 2024, driven by their critical role in assay execution and high repeat purchase rates. Reagents are essential for both clinical diagnostics and research applications, and their consistent quality directly impacts assay accuracy and reliability. Consumables such as plates, pipettes, and microbeads are widely used across hospitals, clinical labs, and research institutes, making this segment indispensable. The dominance is further supported by ongoing innovation in reagents that enhance sensitivity, throughput, and multiplexing capabilities. Laboratories prefer reagent kits from leading providers due to their standardized protocols and ease of integration into existing workflows.
The instruments & accessories segment is expected to witness the fastest growth rate from 2025 to 2032, fueled by increasing automation in laboratories and rising adoption of high-throughput systems. Instruments such as multiplex readers, flow cytometers, and automated sample handling devices allow for faster processing and higher accuracy. The growing demand in clinical and research settings for automated systems that minimize human error and optimize workflow efficiency is driving market expansion. Accessories such as microplates, detection probes, and calibration kits further enhance instrument functionality. Expansion of diagnostic laboratories and research facilities across Saudi Arabia, UAE, and South Africa is accelerating demand for advanced instruments.
- By Type
On the basis of type, the MEA multiplex assays market is segmented into nucleic acid multiplex assays, protein multiplex assays, and cell-based multiplex assays. Protein multiplex assays dominated the market with the largest revenue share of 39% in 2024, owing to their widespread use in biomarker detection, cytokine profiling, and disease research. Protein assays offer high sensitivity and specificity, making them suitable for clinical diagnostics and research applications. The availability of standardized protein assay kits from leading suppliers also contributes to their dominance. Hospitals, clinical labs, and pharmaceutical companies prefer protein assays due to their proven accuracy and ease of integration into workflow pipelines. In addition, protein multiplex assays are increasingly used in immunology and oncology research, further expanding their adoption.
Nucleic acid multiplex assays are expected to witness the fastest growth rate from 2025 to 2032, driven by the increasing demand for rapid and precise genetic and infectious disease diagnostics. PCR-based and real-time nucleic acid assays enable simultaneous detection of multiple pathogens or gene targets, reducing time and cost per test. The rise in infectious disease prevalence in the MEA region and government initiatives for early detection programs are key growth drivers. Research institutes and clinical laboratories increasingly adopt nucleic acid assays due to their high sensitivity, specificity, and ability to integrate with automated platforms. Advances in microfluidics and multiplex PCR technologies are further accelerating growth in this segment.
- By Technology
On the basis of technology, the MEA multiplex assays market is segmented into protein microarray, polymerase chain reaction, multiplex real-time PCR, flow cytometry, fluorescence detection, luminescence, and others. Multiplex real-time PCR dominated the market with the largest revenue share of 36% in 2024, supported by its rapid detection capabilities, high sensitivity, and quantitative output for both clinical and research applications. Its widespread adoption in infectious disease diagnostics and molecular research labs drives its dominance. The ability to detect multiple targets simultaneously while maintaining accuracy makes it a preferred choice for hospitals and laboratories. Leading companies provide multiplex PCR kits compatible with high-throughput instruments, further increasing adoption.
Flow cytometry is expected to witness the fastest growth rate from 2025 to 2032, fueled by its ability to analyze multiple cellular parameters simultaneously, making it critical for immunology, oncology, and cell-based research. The increasing adoption of automated and high-throughput flow cytometers is expanding the applications of this technology. Research institutes and pharmaceutical companies prefer flow cytometry due to its versatility and accuracy. The rising prevalence of chronic and infectious diseases in the MEA region is driving demand for flow cytometry in both diagnostics and R&D. Continuous technological advancements, including multiplex fluorescent labeling, are boosting growth in this segment.
- By Application
On the basis of application, the MEA multiplex assays market is segmented into clinical diagnostics and research & development. Clinical diagnostics dominated the market with the largest revenue share of 44% in 2024, as hospitals and diagnostic laboratories increasingly rely on multiplex assays for simultaneous detection of multiple biomarkers, improving efficiency and reducing costs. The ability to detect several disease markers in a single test enhances patient diagnosis, treatment monitoring, and therapeutic decisions. Government healthcare programs and private diagnostic expansion initiatives further support clinical adoption. The convenience, accuracy, and reproducibility of multiplex assays drive preference over conventional single-analyte tests in clinical settings.
Research & development is expected to witness the fastest growth rate from 2025 to 2032, driven by rising investments in pharmaceutical and biotechnology research, biomarker discovery, and drug development. Multiplex assays allow simultaneous screening of multiple targets, saving time and resources in experimental studies. Academic institutions, research institutes, and pharma companies adopt these assays for preclinical studies, clinical trials, and biomarker validation. Technological advancements in automated platforms and high-throughput screening systems further accelerate adoption in R&D applications. Increasing collaborations between research organizations and multiplex assay providers also contribute to growth.
- By End User
On the basis of end user, the MEA multiplex assays market is segmented into hospitals, clinical laboratories, pharmaceutical & biotechnology companies, research institutes, and others. Hospitals dominated the market with the largest revenue share of 41% in 2024, due to their need for rapid and accurate diagnostic testing for patient management. Hospitals utilize multiplex assays for early detection, disease monitoring, and personalized medicine applications. The presence of advanced diagnostic labs and increasing government support for modern healthcare infrastructure contribute to this dominance. Hospitals also benefit from standardized multiplex assay kits that ensure reliable and reproducible results across different departments.
Pharmaceutical & biotechnology companies are expected to witness the fastest growth rate from 2025 to 2032, fueled by the increasing focus on drug discovery, biomarker validation, and clinical trials. Multiplex assays enable simultaneous analysis of multiple biomarkers, accelerating the drug development process and reducing overall research costs. Companies are adopting these assays for preclinical studies, high-throughput screening, and translational research. Technological advancements, including automated platforms and integration with laboratory information management systems, are further driving adoption. Rising investment in pharmaceutical R&D across the MEA region supports growth in this segment.
Middle East and Africa Multiplex Assays Market Regional Analysis
- South Africa dominated the Middle East and Africa multiplex assays market with the largest revenue share of 38.9% in 2024, characterized by significant healthcare infrastructure investments, strong government initiatives for advanced diagnostics, and presence of leading market players, with the country experiencing substantial growth in laboratory installations and diagnostic testing capabilities
- Healthcare providers and research institutions in the country highly value the efficiency, accuracy, and high-throughput capabilities offered by multiplex assays, enabling simultaneous detection of multiple biomarkers in clinical and research settings
- This widespread adoption is further supported by rising prevalence of chronic and infectious diseases, growing awareness of early diagnosis, and the presence of key market players, establishing multiplex assays as preferred tools for hospitals, clinical laboratories, and research institutes across the region
The South Africa Multiplex Assays Market Insight
The South Africa multiplex assays market captured the largest revenue share of 38.9% in 2024 within the MEA region, fueled by significant investments in healthcare infrastructure and expanding diagnostic and research laboratories. Hospitals and clinical laboratories are increasingly adopting multiplex assays for simultaneous detection of multiple biomarkers, improving efficiency and accuracy in disease diagnostics. The growing prevalence of chronic and infectious diseases, combined with government initiatives for early diagnosis and improved patient care, is further propelling market growth. In addition, the presence of leading global and regional assay providers enhances accessibility to advanced multiplex solutions, supporting widespread adoption across clinical and research applications.
Saudi Arabia Multiplex Assays Market Insight
The Saudi Arabia multiplex assays market is anticipated to grow at the fastest CAGR during the forecast period, driven by increasing healthcare expenditure and modernization of laboratory infrastructure. The country is witnessing rising demand for rapid, accurate diagnostic solutions, particularly for infectious diseases and cancer biomarkers. Government programs supporting national health initiatives, along with private sector investments in hospitals and diagnostic centers, are promoting the uptake of multiplex assays. Technological advancements, including automated high-throughput platforms and integration with laboratory information systems, are enabling faster and more reliable testing. The growing emphasis on research and development in biotechnology and pharmaceutical sectors further contributes to market expansion.
UAE Multiplex Assays Market Insight
The UAE multiplex assays market is projected to expand steadily during the forecast period, driven by increasing healthcare investments and rising adoption of advanced diagnostics in both hospitals and clinical laboratories. The country’s strategic focus on establishing state-of-the-art medical facilities and research centers is boosting the use of multiplex assays for clinical and research purposes. Early disease detection programs and demand for precision medicine applications are encouraging healthcare providers to integrate multiplex testing solutions. The UAE’s strong regulatory framework, combined with collaborations between local and international assay providers, facilitates market growth.
Egypt Multiplex Assays Market Insight
The Egypt multiplex assays market is expected to grow at a noteworthy CAGR during the forecast period, fueled by rising awareness of early diagnosis and increasing prevalence of infectious and chronic diseases. Hospitals and clinical laboratories are gradually adopting multiplex assays to improve testing efficiency and patient outcomes. Investments in healthcare infrastructure and government-led initiatives to expand diagnostic capabilities support market expansion. Furthermore, research institutes are increasingly using multiplex assays for biomarker discovery and disease monitoring, driving demand. The availability of both imported and locally distributed assay kits enhances adoption across clinical and research applications.
Middle East and Africa Multiplex Assays Market Share
The Middle East and Africa Multiplex Assays industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Luminex Corporation (U.S.)
- Illumina, Inc. (U.S.)
- QIAGEN (Netherlands)
- Agilent Technologies, Inc. (U.S.)
- Abcam plc (U.K.)
- BD (U.S.)
- Merck KGaA (Germany)
- PerkinElmer (U.S.)
- Meso Scale Diagnostics, LLC (U.S.)
- Randox Laboratories Ltd. (U.K.)
- Seegene Inc. (South Korea)
- Quanterix Corporation (U.S.)
- Olink Holding AB (Sweden)
- Promega Corporation (U.S.)
- Danaher (U.S.)
- Berthold Technologies GmbH & Co. KG (Germany)
- IBL International GmbH (Germany)
- Crown Bioscience Inc. (U.S.)
What are the Recent Developments in Middle East and Africa Multiplex Assays Market?
- In October 2025, Alamar Biosciences launched the NULISAqpcr™ BD-pTau217 Assay, a transformative leap in blood-based quantification of brain-derived phosphorylated tau 217 (pTau217). This assay is pivotal in Alzheimer's disease research and other tauopathies, offering unparalleled sensitivity and specificity
- In July 2025, Roche and the African Society for Laboratory Medicine (ASLM) launched a partnership to strengthen diagnostic leadership across Africa. This collaboration aims to build long-term leadership in diagnostics, shaping the future of healthcare in Africa
- In May 2025, QIAGEN partnered with ID Solutions to expand its digital PCR oncology research portfolio. This collaboration enhances the QIAcuity platform, enabling faster and more reproducible results for oncology research in the Middle East and Africa
- In February 2024, MGI entered a strategic partnership with Prepaire Labs at MEDLAB Middle East 2024. The collaboration introduced MGI’s DNBSEQ-T20×2 ultra-high throughput sequencer to the region, marking its first application in drug discovery in the Middle East
- In December 2023, Alveo Technologies announced a major distribution expansion across EMEA. The company developed a rapid, handheld, and portable medical-grade platform that pairs advanced molecular assays with cloud-enabled data analytics for real-time analysis and diagnosis of disease and pathogens
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.