Middle East And Africa Hereditary Transthyretin Amyloidosis Market
Market Size in USD Million
CAGR :
% 
 USD
54.94 Million
USD
70.14 Million
2024
2032
USD
54.94 Million
USD
70.14 Million
2024
2032
| 2025 –2032 | |
| USD 54.94 Million | |
| USD 70.14 Million | |
|
|
|
|
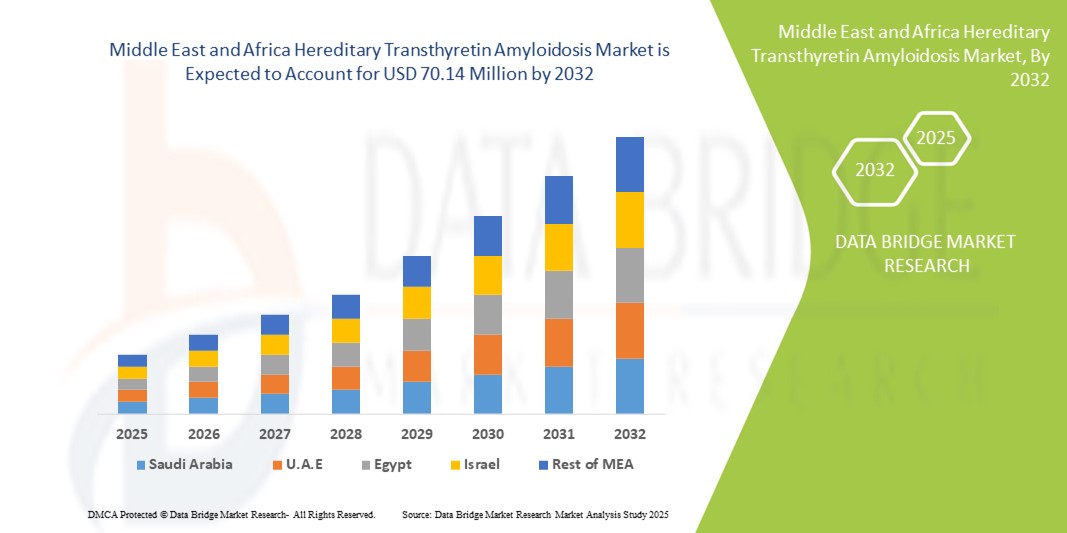
Middle East and Africa Hereditary Transthyretin Amyloidosis Market Size
- The Middle East and Africa hereditary transthyretin amyloidosis market size was valued at USD 54.94 million in 2024 and is expected to reach USD 70.14 million by 2032, at a CAGR of 3.10% during the forecast period
- The market growth is largely fueled by the increasing prevalence of hereditary transthyretin amyloidosis (hATTR) and growing awareness among healthcare professionals and patients about available diagnostic and therapeutic options. Early detection and timely intervention are becoming critical factors in improving patient outcomes
- Furthermore, advancements in molecular diagnostics, genetic testing, and novel therapeutics, including TTR stabilizers and RNA-targeted therapies, are expanding the scope of effective management for patients with hATTR. These innovations are driving the uptake of Hereditary Transthyretin Amyloidosis solutions, thereby significantly boosting the industry's growth
Middle East and Africa Hereditary Transthyretin Amyloidosis Market Analysis
- The Middle East and Africa Hereditary Transthyretin Amyloidosis market is experiencing robust growth, driven by increasing awareness of rare diseases, expanding healthcare infrastructure, and the availability of advanced therapies across the region
- The rising prevalence of hereditary transthyretin amyloidosis (hATTR) and improved diagnostic capabilities in major healthcare centers are further propelling market adoption in the region
- Saudi Arabia dominated the hereditary transthyretin amyloidosis market in the Middle East & Africa with the largest revenue share of 39.1% in 2024, driven by strong investments in healthcare infrastructure, widespread availability of diagnostic centers, and collaborations with international pharmaceutical companies. The country’s Vision 2030 strategy, which emphasizes healthcare modernization and rare disease management, has further strengthened market dominance
- The United Arab Emirates is expected to be the fastest-growing country in the Middle East & Africa hereditary transthyretin amyloidosis market during the forecast period, registering a CAGR of 11.7% from 2025 to 2032. Growth is fueled by rising healthcare expenditure, expansion of specialty clinics, increasing patient awareness, and government-led initiatives to integrate advanced diagnostics and therapies into the healthcare system
- The Male segment dominated the hereditary transthyretin amyloidosis market with a revenue share of 54.7% in 2024, as the prevalence of hATTR, particularly cardiomyopathy, is higher among men in key regional populations
Report Scope and Hereditary Transthyretin Amyloidosis Market Segmentation
|
Attributes |
Hereditary Transthyretin Amyloidosis Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Middle East and Africa
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Middle East and Africa Hereditary Transthyretin Amyloidosis Market Trends
Advancements in Diagnostics and Targeted Therapies
- A significant and accelerating trend in the Middle East and Africa hereditary transthyretin amyloidosis market is the increasing adoption of advanced diagnostic tools and targeted therapies, which are improving patient outcomes and disease management across the region
- For instance, new imaging techniques and genetic testing platforms are enabling earlier and more accurate diagnosis of hATTR, allowing clinicians to tailor treatment plans effectively and monitor disease progression in real-time
- Enhanced treatment regimens, including novel RNA-targeted therapies and small molecule stabilizers, are being introduced in major hospitals and specialty clinics across the Middle East and Africa, offering patients improved efficacy and reduced side effects
- The seamless integration of multidisciplinary care programs, combining diagnostics, therapeutics, and patient monitoring, is facilitating comprehensive disease management and improving quality of life for hATTR patients
- Increased collaborations between local healthcare providers and global pharmaceutical companies are accelerating the availability of cutting-edge therapies and clinical trial participation within the region
- Government initiatives, including healthcare modernization programs and rare disease management strategies, are further supporting the growth of the Hereditary Transthyretin Amyloidosis market by ensuring wider access to advanced diagnostics and treatments
- Awareness campaigns for healthcare professionals and patient advocacy programs are expanding understanding of hATTR symptoms, treatment options, and the importance of early intervention, fostering timely diagnosis and therapy adoption
- Overall, the trend toward integrated care, improved diagnostic precision, and access to novel therapies is driving rapid growth in the Middle East and Africa Hereditary Transthyretin Amyloidosis market, positioning the region for continued expansion in patient-centered disease management
Middle East and Africa Hereditary Transthyretin Amyloidosis Market Dynamics
Driver
Growing Need Due to Rising Awareness and Advanced Healthcare Adoption
- The increasing prevalence of hereditary transthyretin amyloidosis (hATTR) across Middle Eastern and African populations, coupled with heightened awareness among healthcare professionals, is a significant driver for the growing demand for advanced diagnostic and therapeutic solutions
- For instance, in June 2023, Pfizer Inc. announced the launch of its enhanced tafamidis therapy program in collaboration with major hospitals in Saudi Arabia and the UAE, aimed at improving early diagnosis and patient management for hereditary transthyretin amyloidosis. Such initiatives by leading companies are expected to accelerate the growth of the Hereditary Transthyretin Amyloidosis market in the forecast period
- As healthcare providers become more aware of the complexities of hATTR, advanced diagnostics and targeted therapies provide improved patient management, disease monitoring, and individualized treatment plans, which are increasingly preferred over conventional methods
- Furthermore, the rising adoption of modern healthcare practices and digital health solutions in hospitals and specialty clinics is promoting the use of innovative therapeutics, facilitating seamless integration of monitoring and treatment strategies
- The availability of new treatment modalities, including RNA-targeted therapies and stabilizing agents, is enhancing treatment outcomes and quality of life for patients, further driving market adoption in both urban and semi-urban healthcare centers
- Growing investments in healthcare infrastructure, particularly in specialized neurology centers and tertiary hospitals, are creating favorable conditions for the adoption of advanced Hereditary Transthyretin Amyloidosis therapies and diagnostics across the region
- Regional collaborations between local healthcare providers and global pharmaceutical companies are accelerating access to cutting-edge treatment options and patient support programs, strengthening the market growth trajectory
- Overall, the combined effect of increasing disease awareness, healthcare modernization, and availability of advanced diagnostics and therapeutics is expected to continue propelling the growth of the Hereditary Transthyretin Amyloidosis market in the Middle East and Africa region
Restraint/Challenge
Concerns Regarding Affordability and Healthcare Access
- The high cost of advanced diagnostics and novel therapies for hATTR remains a significant barrier, especially in price-sensitive markets within the Middle East and Africa, limiting adoption among certain patient populations
- Limited availability of specialized treatment centers and trained medical professionals in specific regions can restrict patient access to the latest diagnostic and therapeutic options, impacting overall market penetration
- Government initiatives, patient assistance programs, and partnerships with international pharmaceutical companies are crucial to address these challenges, enhancing treatment accessibility and affordability across the region
- While some therapies are gradually becoming more cost-effective, the perceived premium of advanced treatments can still hinder widespread adoption among budget-conscious healthcare providers and patients
- Expanding local manufacturing, enhancing healthcare funding, and improving infrastructure for specialty clinics are essential strategies to overcome these barriers, ensuring sustainable growth of the Hereditary Transthyretin Amyloidosis market in the Middle East and Africa
Middle East and Africa Hereditary Transthyretin Amyloidosis Market Scope
The market is segmented on the basis of diagnosis and treatment, gene variation, gender, indication, end user, and distribution channel.
- By Diagnosis and Treatment
On the basis of diagnosis and treatment, the Hereditary Transthyretin Amyloidosis (hATTR) market is segmented into diagnosis and treatment. The treatment segment dominated the largest market revenue share of 46.5% in 2024, driven by increasing adoption of pharmacological therapies such as transthyretin stabilizers, gene silencers, and supportive care measures. Hospitals and specialty clinics are increasingly recommending advanced treatment regimens due to their proven efficacy in slowing disease progression and improving quality of life. The growing awareness among healthcare professionals and patients regarding early intervention strategies also contributes to the segment’s strong performance. Expansion of access programs and ongoing clinical trials further support treatment adoption. Government initiatives in the region promoting rare disease management, combined with collaborations between global pharmaceutical companies and local healthcare providers, enhance patient accessibility. The treatment segment also benefits from technological advancements in drug delivery and monitoring devices.
The diagnosis segment is expected to witness the fastest CAGR of 12.1% from 2025 to 2032, fueled by rising demand for early and accurate detection of hATTR. Increased availability of genetic testing, biomarker identification, and imaging-based diagnostic tools allows for timely intervention. Hospitals and diagnostic centers are adopting advanced techniques for patient stratification, improving treatment outcomes. Awareness campaigns among clinicians and patient advocacy groups further drive demand for early diagnostic solutions. Technological innovations such as high-throughput screening and next-generation sequencing contribute to segment growth. Expansion of specialized neurology and cardiology centers in the Middle East and Africa also facilitates adoption.
- By Gene Variation
On the basis of gene variation, the Hereditary Transthyretin Amyloidosis (hATTR) market is segmented into V30M, V122L, T60A, and Others. The V30M segment accounted for the largest market revenue share of 41.2% in 2024, as this mutation is highly prevalent in certain populations across the Middle East and Africa. V30M is closely associated with polyneuropathy, driving demand for targeted therapies and diagnostic tools. Patients with this mutation often require early intervention, increasing the adoption of pharmacological treatment and monitoring devices. Clinical awareness programs and genetic counseling services also support market growth. Regional healthcare policies promoting rare disease screening contribute to wider detection and treatment adoption. Multidisciplinary care centers ensure that patients receive both diagnostic and therapeutic support.
The V122L segment is projected to register the fastest CAGR of 11.4% during 2025–2032, supported by rising identification of this mutation in populations at risk for cardiomyopathy. Healthcare providers are increasingly focusing on early detection through genetic testing and cardiovascular monitoring. The growth is further supported by the introduction of specialized therapies targeting V122L-related cardiomyopathy. Awareness campaigns and partnerships between pharmaceutical companies and local healthcare institutions enhance accessibility. Technological advances in non-invasive cardiac imaging also facilitate early intervention.
- By Gender
On the basis of gender, the Hereditary Transthyretin Amyloidosis (hATTR) market is segmented into male and female. The Male segment held the largest market revenue share of 54.7% in 2024, as the prevalence of hATTR, particularly cardiomyopathy, is higher among men in key regional populations. Male patients are more likely to require complex pharmacological treatment, driving adoption of both therapies and monitoring solutions. Clinical guidelines recommend closer monitoring for men, which increases demand for specialized diagnostic tools. Awareness initiatives targeting male patients and caregivers also contribute to revenue growth. Hospitals and specialty clinics provide tailored treatment programs to manage disease progression effectively.
The Female segment is expected to witness the fastest CAGR of 10.5% from 2025 to 2032, driven by increased recognition of hATTR in women, earlier diagnosis, and growing inclusion in clinical trials. Women-focused awareness campaigns and diagnostic screenings are gaining traction, boosting demand for treatment and monitoring services. Technological advancements in wearable and home-based monitoring devices further support segment growth. The rising number of female patients diagnosed in the Middle East and Africa encourages healthcare providers to adopt gender-specific treatment approaches.
- By Indication
On the basis of indication, the Hereditary Transthyretin Amyloidosis (hATTR) market is segmented into Cardiomyopathy (ATTR-CM), Polyneuropathy (ATTR-PN), and Mixed Indications. The Cardiomyopathy (ATTR-CM) segment dominated the market with the largest revenue share of 48.3% in 2024, due to the high clinical burden of cardiac complications and increasing hospitalization rates. The need for specialized pharmacological interventions, continuous cardiac monitoring, and early-stage diagnosis drives segment adoption. Hospitals and cardiology centers in the Middle East and Africa are increasingly integrating ATTR-CM management into routine care. The segment also benefits from ongoing clinical research, government healthcare initiatives, and collaborations with global pharmaceutical companies to provide advanced therapies.
The Polyneuropathy (ATTR-PN) segment is projected to register the fastest CAGR of 11.9% during 2025–2032, reflecting a strong and growing focus on the effective management of nerve-related complications associated with hATTR. This growth is supported by increasing patient awareness about the significance of early detection and management of polyneuropathy symptoms, prompting more individuals to seek timely medical intervention. Technological advances in diagnostic tools, such as sophisticated nerve conduction studies and innovative wearable monitoring solutions, are enabling clinicians to track disease progression more accurately and personalize treatment strategies.
- By End User
On the basis of end user, the Hereditary Transthyretin Amyloidosis (hATTR) market is segmented into hospitals and clinics, diagnostic laboratories, radiology centers, academic and research institutes, ambulatory surgical centers, and homecare. The hospitals and clinics segment held the largest market revenue share of 52.6% in 2024, as these facilities provide integrated care for hATTR patients. Multidisciplinary teams, access to advanced therapies, and diagnostic infrastructure make hospitals the primary point of care. Referral networks and specialized centers enhance patient reach. Government healthcare initiatives and partnerships with global pharmaceutical companies further strengthen hospital dominance.
The homecare segment is expected to witness the fastest CAGR of 13.2% from 2025 to 2032, reflecting a significant shift toward patient-centered care and the growing preference for receiving treatments in the comfort of one’s home. This growth is driven by the increasing adoption of at-home infusion therapies, which provide convenience, reduce hospital visits, and improve patient adherence to treatment regimens. The trend is further supported by advancements in remote monitoring technologies and telemedicine solutions, enabling healthcare providers to track patient progress, manage therapy adjustments, and respond to complications in real time. Rising healthcare spending across the Middle East and Africa is facilitating the expansion of homecare services, allowing greater access to specialized treatments for patients with hereditary transthyretin amyloidosis.
- By Distribution Channel
On the basis of distribution channel, the Hereditary Transthyretin Amyloidosis (hATTR) market is segmented into direct tender, third party distributors, hospital pharmacy, retail pharmacy, and others. The hospital pharmacy segment dominated the largest market revenue share of 44.8% in 2024, reflecting its critical role as the primary point for dispensing specialized therapies and advanced diagnostic devices. Hospitals provide controlled environments for handling complex treatments, ensuring proper storage, dosing, and monitoring. Their integrated clinical support allows for close patient supervision, enhancing treatment efficacy and safety. The dominance of hospital pharmacies is further supported by strong relationships with healthcare providers, streamlined procurement processes, and their capacity to offer bundled services such as patient education, follow-up care, and therapy management. The concentration of specialized expertise and resources in hospital settings makes them the preferred channel for both physicians and patients seeking reliable access to hereditary transthyretin amyloidosis therapies.
The third party distributors segment is anticipated to witness the fastest CAGR of 11.6% from 2025 to 2032, driven by the expansion of logistics networks and strategic partnerships with hospitals, clinics, and homecare providers. These distributors improve the availability of branded therapies and diagnostic tools in remote, underserved, and emerging markets, addressing accessibility challenges. Their efficient supply chain management, warehousing capabilities, and cold-chain solutions ensure timely delivery and product integrity. The segment’s growth is further fueled by increasing collaborations with pharmaceutical manufacturers aiming to broaden market reach and optimize distribution efficiency. Enhanced technological solutions, such as track-and-trace systems and digital order management platforms, are streamlining operations and enabling distributors to meet rising patient demand. The combination of operational scalability, geographical penetration, and focus on service quality positions Third Party Distributors as the fastest-growing distribution channel in the Middle East & Africa hereditary transthyretin amyloidosis market.
Middle East and Africa Hereditary Transthyretin Amyloidosis Market Regional Analysis
- Middle East & Africa dominated the hereditary transthyretin amyloidosis market with the largest revenue share in 2024, driven by strong investments in healthcare infrastructure, widespread availability of diagnostic centers, and collaborations with international pharmaceutical companies. The region’s emphasis on healthcare modernization and rare disease management, particularly through Saudi Arabia’s Vision 2030 strategy, has further strengthened market dominance
- Healthcare providers and patients in the region are increasingly adopting advanced diagnostics and therapies for rare diseases, supported by rising awareness, improved accessibility to specialized centers, and government-led initiatives aimed at integrating innovative treatments into national healthcare systems
- This widespread adoption is further supported by increasing healthcare expenditure, growing specialty clinics, and robust collaborations between local hospitals and global pharmaceutical companies, establishing the Middle East & Africa as a key market for Hereditary Transthyretin Amyloidosis
Saudi Arabia Hereditary Transthyretin Amyloidosis Market Insight
The Saudi Arabia hereditary transthyretin amyloidosis market captured the largest revenue share of 39.1% in the Middle East & Africa market in 2024, fueled by substantial healthcare investments, the expansion of diagnostic and specialty centers, and strong collaborations with international pharmaceutical companies. Government initiatives such as Vision 2030, focusing on rare disease management and healthcare modernization, are accelerating the adoption of advanced diagnostics and therapies across the country.
United Arab Emirates Hereditary Transthyretin Amyloidosis Market Insight
The UAE hereditary transthyretin amyloidosis market is expected to be the fastest-growing country in the Middle East & Africa market, registering a CAGR of 11.7% from 2025 to 2032, driven by rising healthcare spending, expansion of specialty clinics, increasing patient awareness, and government-led initiatives integrating advanced diagnostics and therapies into healthcare systems. Enhanced access to innovative treatment options, growing focus on rare disease management, and the development of medical infrastructure further support rapid market growth in the UAE.
Middle East and Africa Hereditary Transthyretin Amyloidosis Market Share
The Hereditary Transthyretin Amyloidosis industry is primarily led by well-established companies, including:
- Pfizer Inc (U.S.)
- GE HealthCare (U.S.)
- Siemens Healthcare AG (Germany)
- Koninklijke Philips N.V. (Netherlands)
- CANON MEDICAL SYSTEMS CORPORATION (Japan)
- Neusoft Corporation (China)
- NIHON KOHDEN CORPORATION (Japan)
- Shimadzu Corporation (Japan)
- Alnylam Pharmaceuticals, Inc. (U.S.)
- SCHILLER (Switzerland)
- Novo Nordisk A/S (Denmark)
- FONAR Corp (U.S.)
- MinFound Medical Systems Co. (China)
Latest Developments in Middle East and Africa Hereditary Transthyretin Amyloidosis Market
- In May 2025, Alnylam Pharmaceuticals announced plans to present the latest clinical data from its transthyretin amyloidosis (TTR) franchise at the Heart Failure 2025 Congress. This initiative highlights the company’s focus on raising awareness about hATTR and sharing advances in therapies to improve patient outcomes across the Middle East and Africa region
- In April 2023, Canon Medical Systems Corporation announced that it had begun clinical research by employing a next-generation X-ray CT system with photon counting computed tomography with the National Cancer Center Japan (NCC) . This will help the organization in developing its products category
- In June 2022, Alnylam Pharmaceuticals, Inc. received FDA authorization for AMVUTTRA (vutrisiran) for the treatment of Polyneuropathy of hereditary transthyretin-mediated amyloidosis
- In December 2022, a new technology AIR Recon DL was recently recognized in the “Best of What's New” awards by Popular Science magazine. It is a new technique developed by GE Healthcare for delivering high-quality images in a short amount of time. AIR Recon DL uses deep-learning technology to simultaneously improve MRI image quality and enable reduced scan time, improving the patient experience
- In August 2020, Siemens Healthcare GmbH entered into a strategic agreement to develop advanced diagnostic and treatment solutions for rare diseases, including hereditary transthyretin amyloidosis. This collaboration aimed to expand the company’s product offerings and strengthen its presence in specialized healthcare segments within the region
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.