Middle East Molecular Point Of Care Testing Using Naat Market
Market Size in USD Million
CAGR :
% 
 USD
274.00 Million
USD
484.16 Million
2024
2032
USD
274.00 Million
USD
484.16 Million
2024
2032
| 2025 –2032 | |
| USD 274.00 Million | |
| USD 484.16 Million | |
|
|
|
|
Middle East Molecular Point of Care Testing (using NAAT) Market Size
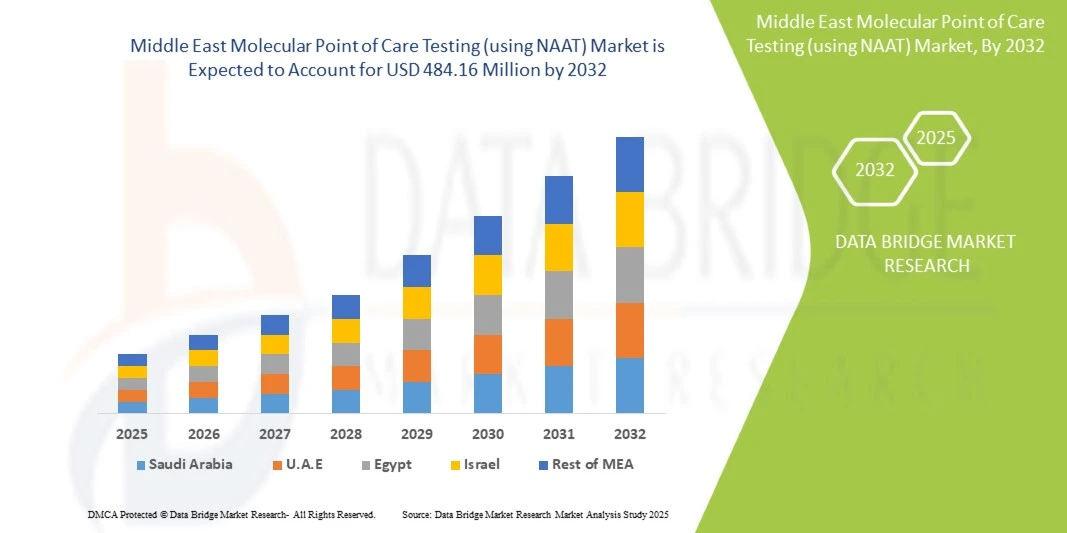
- The Middle East molecular point of care testing (using NAAT) market size was valued at USD 274.00 million in 2024 and is expected to reach USD 484.16 million by 2032, at a CAGR of 7.4% during the forecast period
- The market growth is largely fueled by the increasing prevalence of infectious diseases, technological advancements in molecular diagnostics, and the rising adoption of point-of-care testing across hospitals, clinics, and diagnostic laboratories
- Furthermore, growing healthcare expenditure, government initiatives to improve diagnostic accessibility, and demand for rapid, accurate, and decentralized testing solutions are establishing NAAT-based point-of-care testing as a preferred diagnostic tool in the region. These converging factors are accelerating the uptake of molecular point-of-care solutions, thereby significantly boosting the industry's growth
Middle East Molecular Point of Care Testing (using NAAT) Market Analysis
- Molecular point-of-care testing using NAAT, offering rapid and accurate detection of infectious diseases at or near the site of patient care, is increasingly vital in modern healthcare systems across hospitals, clinics, and diagnostic laboratories due to its high sensitivity, quick turnaround time, and ease of integration into clinical workflows
- The escalating demand for NAAT-based point-of-care testing is primarily fueled by the rising prevalence of infectious diseases, growing awareness of early diagnostics, and a preference for decentralized, rapid testing solutions over traditional laboratory testing
- Saudi Arabia dominated the Middle East NAAT-based point-of-care testing market with the largest revenue share of 38.5% in 2024, characterized by advanced healthcare infrastructure, high healthcare expenditure, and a strong presence of key industry players, with substantial growth driven by innovations from both established diagnostic companies and emerging local startups
- United Arab Emirates is expected to be the fastest-growing country in the market during the forecast period, due to increasing healthcare investments, government initiatives to improve diagnostic access, and rising adoption of modern diagnostic technologies
- The Respiratory Infections Testing segment dominated the market with a share of 50.5% in 2024, driven by the high prevalence of viral and bacterial infections and the established clinical reliability of NAAT technology for rapid and accurate detection
Report Scope and Middle East Molecular Point of Care Testing (using NAAT) Market Segmentation
|
Attributes |
Middle East Molecular Point of Care Testing (using NAAT) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Middle East Molecular Point of Care Testing (using NAAT) Market Trends
Rapid, Decentralized Testing for Infectious Diseases
- A significant and accelerating trend in the Middle East NAAT-based point-of-care testing market is the growing adoption of rapid, decentralized diagnostic solutions that can deliver highly accurate results near the patient care site, reducing dependency on central laboratories
- For instance, the Cepheid GeneXpert platform is widely deployed in hospitals and clinics across Saudi Arabia and UAE, providing near-patient results for tuberculosis, COVID-19, and sexually transmitted infections. Similarly, Abbott ID NOW devices are being implemented in community healthcare settings for fast influenza and COVID-19 detection
- Integration with digital health platforms and laboratory information systems enables real-time reporting, automated alerts, and improved patient management. For instance, NAAT POCT devices connected to hospital EMR systems in the UAE allow clinicians to receive instant notifications of positive cases, facilitating faster intervention
- The trend toward portable, user-friendly, and multiplex NAAT testing devices is driving broader accessibility, enabling clinics and even remote testing centers to perform rapid diagnostics for multiple infectious diseases
- This shift toward rapid, accurate, and decentralized testing is reshaping diagnostic expectations in the region. Companies such as bioMérieux and QIAGEN are developing compact, point-of-care NAAT devices with integrated reporting and remote monitoring capabilities, improving the speed and reliability of infectious disease detection
- The demand for rapid, decentralized, and digitally integrated molecular POCT solutions is growing across hospitals, clinics, and community testing centers, as healthcare providers increasingly prioritize faster diagnosis and better patient management
Middle East Molecular Point of Care Testing (using NAAT) Market Dynamics
Driver
Rising Prevalence of Infectious Diseases and Healthcare Investments
- The increasing burden of infectious diseases such as COVID-19, influenza, tuberculosis, and sexually transmitted infections in Middle Eastern countries, coupled with growing healthcare infrastructure investments, is a significant driver for the rising adoption of NAAT-based point-of-care testing
- For instance, in 2024, the Saudi Ministry of Health expanded its rapid testing programs across hospitals and primary healthcare centers, deploying NAAT devices for early detection of COVID-19 and respiratory infections. Such initiatives by governments and healthcare organizations are expected to drive market growth during the forecast period
- As clinicians and public health authorities prioritize timely diagnosis and effective patient management, NAAT POCT solutions offer rapid turnaround, high sensitivity, and accurate detection, providing a compelling alternative to traditional centralized laboratory testing
- Furthermore, increasing adoption of electronic health records, telemedicine, and digital health platforms is creating a favorable environment for integrated POCT solutions, enabling seamless data management and real-time reporting
- The need for rapid, accurate, and decentralized diagnostic solutions, coupled with government support and investments in healthcare infrastructure, is propelling the adoption of NAAT-based point-of-care testing in both public and private healthcare sectors
Restraint/Challenge
High Costs and Limited Skilled Workforce
- The relatively high cost of NAAT-based POCT devices, consumables, and reagents remains a significant challenge for broader market penetration, particularly in smaller clinics and budget-constrained hospitals. While prices are gradually decreasing, the upfront investment for advanced molecular testing devices can hinder adoption
- For instance, smaller hospitals in Egypt and Qatar have reported budget limitations when procuring advanced NAAT platforms from companies such as Cepheid and Abbott, limiting widespread deployment
- Another key challenge is the shortage of trained laboratory and clinical personnel capable of operating NAAT devices accurately and interpreting results. Mismanagement or improper handling can affect test reliability and patient outcomes
- Addressing these challenges through training programs, government subsidies, and cost-effective NAAT platforms will be vital for sustained market growth. Companies such as bioMérieux and QIAGEN are emphasizing user-friendly interfaces, remote training, and support to enhance adoption in resource-limited settings
- Overcoming high costs and workforce limitations through innovative, easy-to-use, and affordable NAAT solutions will be critical for expanding access and driving long-term market growth in the Middle East
Middle East Molecular Point of Care Testing (using NAAT) Market Scope
The market is segmented on the basis of product, indication, end user, mode of testing, and distribution channel.
- By Product
On the basis of product, the market is segmented into instruments and consumables & reagents. Instruments dominated the market in 2024 with the largest revenue share due to their critical role in NAAT-based testing and high upfront cost. Hospitals, clinics, and reference laboratories prefer investing in advanced instruments to ensure accurate and reliable results. Instruments can support multiple assays and multiplex testing, allowing efficient handling of respiratory, gastrointestinal, and STI diagnostics. Governments and healthcare providers in Saudi Arabia and UAE are deploying these devices in public health programs to improve diagnostic turnaround. Their durability, automation, and integration with digital health platforms make them indispensable in hospital workflows. Major companies such as Cepheid, Abbott, and bioMérieux provide compact, automated instruments designed for near-patient testing, further boosting their adoption.
Consumables & Reagents are expected to witness the fastest growth from 2025 to 2033, driven by recurring demand for test cartridges, extraction kits, and reagents with every test performed. The rising prevalence of infectious diseases and expansion of community testing centers fuel demand. Multiplex reagents that can test for multiple pathogens simultaneously improve cost-efficiency and appeal to healthcare providers. Emerging clinics and smaller hospitals prefer these consumables for flexible and rapid testing without investing in multiple instruments. Increasing government-led screening campaigns and telehealth integration further enhance adoption. The growth of these consumables ensures steady revenue streams for diagnostic companies.
- By Indication
On the basis of indication, the market is segmented into respiratory infections testing, sexually transmitted infection (STI) testing, gastrointestinal tract infections testing, and others. Respiratory Infections Testing dominated the market in 2024 due to the high prevalence of diseases such as COVID-19, influenza, and RSV across the Middle East. Hospitals and clinics rely on rapid NAAT testing for timely patient management and infection control. Governments in Saudi Arabia and UAE have launched mass screening initiatives, further boosting demand. NAAT’s high sensitivity and quick turnaround make it preferable over antigen or culture-based methods. The segment’s dominance is reinforced by seasonal outbreaks and pandemic preparedness measures. Healthcare providers prioritize respiratory testing as it directly impacts treatment decisions and hospital workflow efficiency.
Sexually Transmitted Infection (STI) Testing is expected to witness the fastest growth from 2025 to 2033 due to rising awareness and government-supported screening programs. NAAT-based STI tests provide high sensitivity and specificity for chlamydia, gonorrhea, and other STIs, making them ideal for near-patient diagnostics. Increasing adoption in clinics and ambulatory centers for discreet and rapid testing drives expansion. The growing prevalence of STIs in urban populations and rising demand for private testing solutions support this segment’s fast growth. Multiplex NAAT panels for STI detection further enhance efficiency and convenience. The expansion of telehealth and home-based sample collection also fuels adoption of NAAT for STIs.
- By End User
On the basis of end user, the market is segmented into laboratories, hospitals, clinics, ambulatory centers, homecare, assisted living facilities, and others. Hospitals dominated the market in 2024 due to high patient volumes and the need for fast, reliable diagnostics. Hospitals benefit from in-house NAAT instruments that reduce diagnostic delays and improve infection control. Deployment in emergency, inpatient, and outpatient departments ensures continuous demand. Government and private hospitals in Saudi Arabia and UAE adopt NAAT POCT for national screening programs. The combination of patient throughput, clinical requirement, and infrastructure investment makes hospitals the largest revenue contributor. Hospitals also leverage integrated reporting systems for real-time monitoring of infectious disease trends.
Clinics are expected to be the fastest-growing end-user segment from 2025 to 2033, driven by decentralization of testing and patient demand for near-patient diagnostics. Clinics can provide same-day results, reducing referrals to central laboratories and improving patient satisfaction. Growth of primary healthcare centers and community clinics in UAE, Egypt, and Qatar is boosting adoption. Clinics prefer compact, user-friendly NAAT devices that require minimal staff training. The trend of preventive health checkups and routine infectious disease screening further accelerates growth. Increasing integration with telemedicine platforms enhances the convenience and scalability of POCT in clinics.
- By Mode of Testing
On the basis of mode of testing, the market is segmented into prescription-based testing and OTC testing. Prescription-Based Testing dominated the market in 2024 due to medical supervision requirements for NAAT testing and regulatory compliance. Hospitals and clinics provide prescription-based tests to ensure accurate handling and interpretation. Regulatory policies in Saudi Arabia, UAE, and Egypt mandate professional oversight for molecular diagnostics. High cost and specialized training further support prescription-based dominance. This approach ensures quality control and reduces the risk of misdiagnosis. Most large-scale screening and hospital deployments rely on prescription-based NAAT solutions.
OTC Testing is expected to witness the fastest growth from 2025 to 2033 due to rising demand for home-based diagnostics and telehealth integration. Patients can perform NAAT tests for COVID-19, influenza, and other infections at home and share results with healthcare providers remotely. This approach increases accessibility and convenience, particularly in urban areas with busy lifestyles. Digital platforms enable remote guidance and reporting, further supporting adoption. Growing awareness of preventive healthcare and self-testing drives expansion in this segment. OTC NAAT testing provides flexibility for patients while easing pressure on hospitals and clinics.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. Hospital Pharmacy dominated the market in 2024 due to centralized procurement for hospitals and clinics. Hospital pharmacies manage supply of instruments, test kits, and consumables efficiently across multiple departments. Bulk procurement ensures cost-effectiveness and steady supply of reagents. Hospitals rely on pharmacy-managed logistics to maintain uninterrupted testing services. Integration with hospital IT systems enables inventory management and real-time replenishment. Hospital pharmacies remain the primary revenue channel for NAAT POCT companies in the region.
Online Pharmacy is expected to witness the fastest growth from 2025 to 2033 as digital health adoption expands in the Middle East. Patients can order NAAT kits for home testing with delivery services, benefiting from convenience and discreet access. Telehealth platforms support remote guidance and result submission. Growth in e-commerce and mobile health apps accelerates this channel’s adoption. Home testing and remote monitoring enhance patient engagement. Online pharmacies provide accessibility to both urban and remote populations, making it a rapidly expanding distribution segment.
Middle East Molecular Point of Care Testing (using NAAT) Market Regional Analysis
- Saudi Arabia dominated the Middle East NAAT-based point-of-care testing market with the largest revenue share of 38.5% in 2024, characterized by advanced healthcare infrastructure, high healthcare expenditure, and a strong presence of key industry players, with substantial growth driven by innovations from both established diagnostic companies and emerging local startups
- Healthcare providers and public health authorities in Saudi Arabia highly value the accuracy, speed, and reliability of NAAT-based POCT for managing respiratory infections, STIs, and other infectious diseases. Rapid diagnosis helps in timely treatment, outbreak containment, and improved patient outcomes
- This widespread adoption is further supported by high healthcare expenditure, a technologically advanced medical workforce, and strong partnerships with leading global diagnostic companies. Hospitals, clinics, and laboratories increasingly integrate NAAT POCT devices with hospital information systems, enabling real-time reporting and efficient patient management
The Saudi Arabia Molecular Point of Care Testing (using NAAT) Market Insight
The Saudi Arabia molecular point-of-care testing (using NAAT) market captured the largest revenue share of 38.5% in 2024, driven by the high prevalence of infectious diseases and advanced healthcare infrastructure. Hospitals, clinics, and laboratories are increasingly adopting NAAT-based diagnostics for rapid, accurate detection of respiratory infections, STIs, and gastrointestinal infections. The government’s national screening programs and pandemic preparedness initiatives further boost adoption. Rising investments in healthcare technology and the integration of NAAT devices with digital health platforms, including hospital information systems and telemedicine, are expanding market reach. The increasing focus on preventive healthcare and infection control measures continues to stimulate market growth.
United Arab Emirates Molecular Point of Care Testing (using NAAT) Market Insight
The UAE molecular point-of-care testing market is emerging as the fastest-growing country in the region due to rising healthcare investments and expanding hospital networks. Adoption of NAAT-based testing is supported by government programs for infectious disease surveillance and telehealth integration. Clinics and hospitals are embracing rapid, near-patient testing for timely diagnosis and improved patient management. The UAE’s technologically advanced healthcare system, coupled with high patient awareness of early diagnostics, is driving market growth. The growing demand for multiplex NAAT testing and homecare solutions also contributes to adoption.
Egypt Molecular Point of Care Testing (using NAAT) Market Insight
The Egypt NAAT-based POCT market accounted for a significant share in 2024, driven by improving healthcare infrastructure and rising awareness of rapid molecular diagnostics. Hospitals and primary healthcare centers increasingly adopt NAAT tests for respiratory infections and STIs. Government initiatives supporting decentralized testing and community-based screening programs strengthen market growth. The expansion of telemedicine platforms and digital reporting systems enhances accessibility and convenience. Increasing prevalence of infectious diseases and rising patient demand for faster diagnosis support the market. Affordability and local partnerships with diagnostic companies are further stimulating adoption.
Qatar Molecular Point of Care Testing (using NAAT) Market Insight
The Qatar NAAT point-of-care testing market is witnessing steady growth due to government-backed healthcare programs and a growing emphasis on preventive diagnostics. Hospitals and clinics are adopting NAAT devices for infectious disease screening and routine monitoring. High per capita healthcare expenditure, coupled with strong healthcare infrastructure, drives adoption of advanced molecular diagnostics. The use of NAAT for rapid diagnosis in emergency and outpatient settings further supports market expansion. Integration with hospital IT systems allows real-time reporting and efficient patient management. Growing awareness of early diagnostics among patients encourages utilization in both public and private healthcare sectors.
Middle East Molecular Point of Care Testing (using NAAT) Market Share
The Middle East Molecular Point of Care Testing (using NAAT) industry is primarily led by well-established companies, including:
- Abbott (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- BIOMÉRIEUX (France)
- QIAGEN (Netherlands)
- Danaher (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- BD (U.S.)
- Illumina, Inc. (U.S.)
- Siemens Healthineers AG (Germany)
- Grifols, S.A. (Spain)
- QuidelOrtho Corporation (U.S.)
- DiaSorin S.p.A. (Italy)
- Sysmex Corporation (Japan)
- Sekisui Diagnostics, LLC (U.S.)
- Hologic, Inc. (U.S.)
- Medtronic (Ireland)
- Atlas Medical GmbH (Germany)
- Nova Biomedical (U.S.)
- Werfen (Spain)
What are the Recent Developments in Middle East Molecular Point of Care Testing (using NAAT) Market?
- In February 2025, Aptitude received Emergency Use Authorization (EUA) from the U.S. FDA for its Metrix COVID/Flu multiplex test. This test provides PCR-grade accuracy for the detection and differentiation of SARS-CoV-2, Influenza A, and Influenza B in approximately 20 minutes at the point of care
- In January 2025, Roche received FDA clearance with CLIA waiver for its cobas liat molecular tests to aid in diagnosing sexually transmitted infections (STIs) at the point of care. The tests, which provide results in 20 minutes, enable clinicians to diagnose and differentiate between multiple STIs with a single sample using gold-standard PCR technology
- In October 2024, Access Genetics (doing business as OralDNA® Labs) acquired Sensible Diagnostics Inc. The acquisition's goal is to commercialize a new point-of-care molecular diagnostics platform that aims to deliver central lab-quality test results from saliva or swabs in under 10 minutes, enhancing the speed and affordability of diagnostics
- In September 2024, QIAGEN expanded its strategic partnership with Brazil's Bio-Manguinhos/Fiocruz. The collaboration focuses on providing advanced PCR-based molecular screening platforms for diseases such as malaria, HIV, and hepatitis B and C within Brazil's national blood donation program
- In May 2024, bioMérieux announced a strategic partnership with AnaBioTec, a Belgian analytical service provider. The collaboration is focused on transforming Mycoplasma testing for the biopharmaceutical and cell and gene therapy industries by using bioMérieux's fully automated PCR system to provide a faster, more accurate in-house solution
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.