North America Pdx Models Market
Market Size in USD Billion
CAGR :
% 
 USD
103.42 Billion
USD
370.69 Billion
2024
2032
USD
103.42 Billion
USD
370.69 Billion
2024
2032
| 2025 –2032 | |
| USD 103.42 Billion | |
| USD 370.69 Billion | |
|
|
|
|
North America Patient Derived Xenograft (PDX) Models Market Size
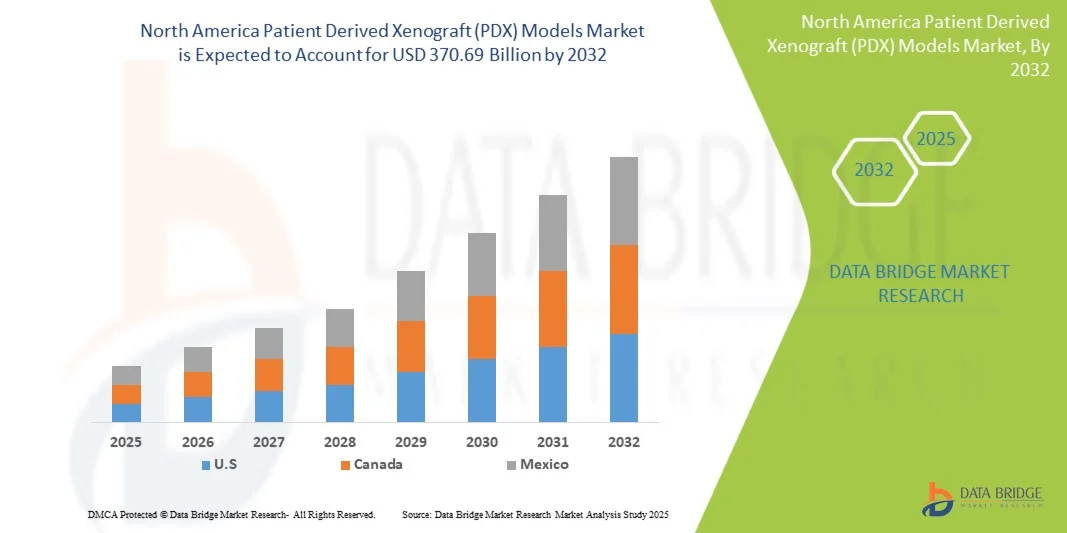
- The North America Patient Derived Xenograft (PDX) models market size was valued at USD 103.42 billion in 2024 and is expected to reach USD 370.69 billion by 2032, at a CAGR of 17.30% during the forecast period
- The market growth is largely fueled by the increasing prevalence of cancer, rising demand for personalized medicine, and the need for more predictive preclinical models, which are enhancing the efficiency and success rates of drug development in oncology
- Furthermore, strong research infrastructure, significant investments in oncology R&D, and the high presence of leading PDX model providers in North America are driving adoption, establishing PDX models as a preferred choice for preclinical cancer research. These converging factors are accelerating the uptake of PDX models, thereby significantly boosting the industry's growth
North America Patient Derived Xenograft (PDX) Models Market Analysis
- Patient-Derived Xenograft (PDX) models, providing in vivo platforms for testing human tumor responses, are increasingly vital components of preclinical oncology research in both academic and pharmaceutical settings due to their high translational relevance, predictive accuracy, and ability to mimic patient-specific tumor biology
- The escalating demand for PDX models is primarily fueled by the rising prevalence of cancer, growing focus on personalized medicine, and the need for more predictive preclinical models to improve drug development success rates
- The United States dominated the PDX models market with the largest revenue share of 42.3% in 2024, characterized by a robust biopharmaceutical industry, advanced research infrastructure, and substantial investments in oncology R&D, with widespread adoption of PDX models in preclinical studies driven by both established pharmaceutical companies and specialized model providers
- Canada is expected to be the fastest growing country in the PDX models market during the forecast period due to increasing oncology research initiatives, growing biotechnology investments, and expanding clinical and preclinical infrastructure
- Mice models segment dominated the PDX models market with a market share of 52.8% in 2024, driven by their established utility for tumor engraftment, ease of handling, and strong reproducibility in preclinical studies
Report Scope and North America Patient Derived Xenograft (PDX) Models Market Segmentation
|
Attributes |
North America Patient Derived Xenograft (PDX) Models Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Patient Derived Xenograft (PDX) Models Market Trends
Advancement Through Humanized and Immuno-Oncology Models
- A significant and accelerating trend in the North America PDX models market is the development of humanized and immuno-oncology compatible PDX models, which provide more clinically relevant platforms for testing tumor-immune interactions and personalized cancer therapies
- For instance, some PDX providers have engineered humanized mouse models that allow engraftment of human immune cells alongside patient tumors, enabling more accurate evaluation of immunotherapies and combination treatments
- Integration of high-throughput genomic profiling with PDX models enables researchers to identify patient-specific mutations and drug responses, accelerating precision oncology drug development and optimizing preclinical study design
- The seamless combination of PDX models with digital data platforms and AI-driven analytics facilitates the identification of biomarkers, therapy resistance mechanisms, and potential drug targets, creating a unified workflow for translational cancer research
- This trend towards more predictive, personalized, and immunologically relevant PDX models is fundamentally reshaping preclinical oncology research, with companies such as Champions Oncology developing humanized PDX models to support advanced immunotherapy testing
- The demand for PDX models that offer humanized immune system compatibility and integrated genomic profiling is growing rapidly across both pharmaceutical and academic research sectors, as precision medicine becomes the cornerstone of oncology drug development
North America Patient Derived Xenograft (PDX) Models Market Dynamics
Driver
Rising Demand for Personalized Medicine and Preclinical Accuracy
- The increasing prevalence of cancer, coupled with the growing focus on personalized medicine, is a significant driver for the heightened adoption of PDX models in drug discovery and development
- For instance, in 2024, The Jackson Laboratory reported expansion of its PDX repository to include rare tumor subtypes, providing researchers with enhanced preclinical testing options
- PDX models offer higher translational relevance compared to traditional cell line models, allowing more accurate prediction of clinical efficacy and patient response, which is critical for reducing late-stage drug development failures
- Furthermore, the growing investment by pharmaceutical companies and research institutions in oncology R&D is driving the integration of PDX models into preclinical pipelines to streamline drug testing and optimize dosing strategies
- The combination of personalized tumor profiling, predictive preclinical modeling, and high reproducibility is propelling PDX adoption, enabling researchers to make data-driven decisions and accelerate therapeutic innovation in oncology
Restraint/Challenge
High Cost and Ethical Considerations Limiting Adoption
- The relatively high cost of establishing and maintaining PDX models, including specialized housing, immunodeficient mice, and tumor engraftment procedures, poses a significant barrier to widespread adoption
- For instance, smaller biotech firms and academic labs may be hesitant to invest in PDX models due to the financial burden associated with long-term study timelines and complex experimental protocols
- Ethical concerns regarding the use of animals in research, coupled with strict regulatory compliance requirements for animal welfare, present challenges to expansion and operational scalability in the PDX models market
- Addressing these challenges through cost-effective PDX platforms, standardized protocols, and adherence to ethical guidelines is crucial for encouraging broader utilization of these models
- While humanized and advanced PDX models provide superior predictive accuracy, the combination of high costs and ethical considerations can hinder adoption, particularly among early-stage researchers and institutions with limited funding
- Overcoming these challenges through collaborative research programs, government grants, and the development of alternative models will be vital for sustained growth and increased penetration of PDX models in North America
North America Patient Derived Xenograft (PDX) Models Market Scope
The market is segmented on the basis of type, tumor type, application, technique, and end user.
- By Type
On the basis of type, the PDX models market is segmented into mice models and rat models. The mice models segment dominated the market with the largest revenue share of 52.8% in 2024, driven by their extensive use in oncology research and preclinical drug development. Mice models are preferred due to their small size, ease of handling, and rapid breeding cycles, which allow for longitudinal studies and faster experimentation. Their well-characterized immune systems and availability of humanized variants make them ideal for evaluating tumor responses to novel therapies. Researchers also favor mice models for high reproducibility and cost efficiency in large-scale studies. The strong adoption of mice models is further supported by advanced genetic modification techniques that enhance predictive accuracy for patient-specific tumors. Overall, their versatility across multiple tumor types and applications cements their dominant position in the market.
Rat models segment is anticipated to witness the fastest growth during the forecast period, fueled by increased use in co-clinical trials and precision medicine research. Rat models offer larger tumor volumes, which facilitate surgical manipulations and detailed imaging studies. Their physiology allows better pharmacokinetic and pharmacodynamic evaluations compared to smaller models. Integration with advanced biomarker analysis and imaging technologies increases their research utility. Pharmaceutical companies are increasingly adopting rat models for immuno-oncology and metastatic studies. These advantages collectively drive faster adoption and market expansion for rat models.
- By Tumor Type
On the basis of tumor type, the market is segmented into respiratory tumor models, urological tumor models, lung tumor models, gastrointestinal tumor models, hematological tumor models, gynecological tumor models, and others. Gastrointestinal tumor models dominated the market in 2024 due to the high prevalence of colorectal, gastric, and pancreatic cancers. These models allow researchers to evaluate tumor heterogeneity, drug response, and therapy resistance in a controlled preclinical environment. They are extensively used in both academic and pharmaceutical studies for screening anticancer compounds. High reproducibility and translational relevance to human disease drive their adoption. In addition, gastrointestinal PDX models support biomarker discovery and validation for targeted therapies. Their combination with molecular profiling enhances precision medicine research, solidifying their dominant market position.
Hematological tumor models are expected to witness the fastest growth during the forecast period due to rising research in leukemia, lymphoma, and myeloma therapies. These models enable detailed studies of bone marrow engraftment, immune interactions, and tumor progression. They are increasingly used to evaluate novel immunotherapies and combination regimens. The rising incidence of blood cancers globally fuels demand for these models. Integration with co-clinical trial protocols further accelerates adoption. Their high translational accuracy positions hematological models as a rapidly expanding subsegment in North America.
- By Application
On the basis of application, the market is segmented into preclinical drug development and oncology research, precision medicine, co-clinical trials, basic cancer research, and biomarker analysis. Preclinical drug development and oncology research dominated the market in 2024, driven by the adoption of PDX models for evaluating the efficacy and safety of new therapeutics before clinical trials. These models reduce translational gaps and enhance the predictability of human responses. They allow researchers to test multiple drug combinations efficiently. Pharmaceutical companies rely on these models for dose optimization and early-stage decision-making. High reproducibility and relevance to human tumors make them indispensable. Their widespread integration into drug pipelines reinforces their dominance in this segment.
Precision medicine segment is anticipated to witness the fastest growth during the forecast period, fueled by the increasing need for patient-specific tumor models to guide therapy selection. Integration of genomic profiling with PDX models enables individualized treatment strategies. Researchers can study drug sensitivity and resistance patterns in a clinically relevant context. Co-clinical studies further enhance personalized treatment validation. Growing investments in oncology precision medicine support the expansion of this segment. The ability to replicate patient-specific tumors makes precision medicine applications a rapidly growing area in North America.
- By Technique
On the basis of technique, the market is segmented into heterotopic implantation and orthotopic implantation. Heterotopic implantation dominated the market in 2024 due to procedural simplicity, reproducibility, and suitability for high-throughput drug screening. Subcutaneous implantation allows easy tumor measurement, sampling, and monitoring. Researchers benefit from standardized protocols that reduce variability across experiments. The technique supports multiple tumor types and facilitates early-phase drug evaluation. Cost-effectiveness and ease of use make it attractive for both CROs and academic institutions. These advantages have established heterotopic implantation as the dominant technique in the region.
Orthotopic implantation is expected to witness the fastest growth during the forecast period because it better mimics the tumor microenvironment at the organ-specific site. This provides higher predictive accuracy for metastasis and therapeutic response. Researchers can study tumor-host interactions more precisely. It is particularly useful for evaluating advanced therapeutics and metastatic progression. Increasing adoption by pharmaceutical companies for translational oncology studies is driving growth. Orthotopic models’ ability to replicate human tumor biology ensures accelerated market expansion.
- By End User
On the basis of end user, the market is segmented into contract research organizations (CROs), academic and research institutions, pharmaceutical and biotechnology companies, and others. Academic and research institutions dominated the market in 2024, due to their extensive utilization of PDX models for basic cancer research, tumor biology studies, and experimental therapeutic evaluations. They have the infrastructure and expertise to handle complex PDX studies. Collaboration with pharmaceutical companies enhances translational research potential. High adoption is also supported by grant funding and research initiatives. Access to advanced facilities and trained personnel ensures sustained dominance. These factors collectively drive the subsegment’s leadership in North America.
Pharmaceutical and biotechnology companies are expected to witness the fastest growth during the forecast period, driven by increasing reliance on PDX models for preclinical drug development, co-clinical trials, and precision oncology pipelines. They use PDX models to evaluate novel compounds, optimize dosing, and improve clinical trial success rates. Integration with biomarker discovery accelerates development timelines. Rising investment in oncology pipelines supports expansion. Adoption of humanized and genetically engineered PDX models enhances predictive power. These factors collectively fuel rapid growth in this subsegment.
North America Patient Derived Xenograft (PDX) Models Market Regional Analysis
- The U.S. dominated the PDX models market with the largest revenue share of 42.3% in 2024, characterized by a robust biopharmaceutical industry, advanced research infrastructure, and substantial investments in oncology R&D, with widespread adoption of PDX models in preclinical studies driven by both established pharmaceutical companies and specialized model providers
- Researchers and pharmaceutical companies in the region highly value the translational relevance, predictive accuracy, and patient-specific modeling offered by PDX models, which enable more effective preclinical drug development and precision oncology studies
- This widespread adoption is further supported by well-established contract research organizations, academic institutions with advanced capabilities, and the growing demand for personalized medicine, establishing PDX models as a preferred platform for oncology research and drug discovery in both clinical and preclinical settings
Canada PDX Models Market Insight
The Canada PDX models market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing government funding in oncology research and rising investments in biotechnology. The growing demand for predictive preclinical models in both academic institutions and pharmaceutical companies fosters adoption. Canadian researchers are increasingly integrating PDX models with genomic profiling and biomarker analysis to support precision medicine. The country’s well-developed healthcare infrastructure, coupled with a focus on translational oncology research, promotes the utilization of PDX models across multiple tumor types. In addition, collaborations between academic institutions and biotech firms are further stimulating market growth.
Mexico PDX Models Market Insight
The Mexico PDX models market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising awareness of personalized cancer research and the expansion of contract research services. Increased government initiatives to promote biotechnology research and oncology studies are encouraging adoption of PDX models. Academic institutions and emerging pharmaceutical companies in Mexico are increasingly using PDX models for preclinical drug evaluation and biomarker discovery. The growing presence of CROs providing PDX model services enhances accessibility and scalability for research studies. Moreover, the focus on improving cancer treatment outcomes is expected to sustain market growth.
North America Patient Derived Xenograft (PDX) Models Market Share
The North America Patient Derived Xenograft (PDX) Models industry is primarily led by well-established companies, including:
- Charles River Laboratories (U.S.)
- Crown Bioscience (U.S.)
- Oncodesign Services (France)
- THE JACKSON LABORATORY (U.S.)
- Altogen Labs (U.S.)
- Inotiv. (U.S.)
- WuXi AppTec (U.S.)
- Hera Biolabs (U.S.)
- XenTech (France)
- Abnova Corporation (Taiwan)
- NexusPharma (U.S.)
- Mediford Corporation (U.S.)
- DarwinHealth, Inc. (U.S.)
- Taconic Biosciences, Inc. (U.S.)
- Champions Oncology, Inc. (U.S.)
- Labcorp Drug Development (U.S.)
- Xenopat (Spain)
What are the Recent Developments in North America Patient Derived Xenograft (PDX) Models Market?
- In March 2025, Mediford Corporation expanded its services to include the establishment and banking of PDX-derived cell lines, along with conducting pharmacological studies at its GLP-certified Kumamoto Laboratories. This expansion aims to provide researchers with more predictive and clinically relevant models for drug development and cancer research
- In November 2024, the UCL Cancer Institute announced the deposition of 44 new PDX models derived from multiple regions of primary patient non-small cell lung cancer (NSCLC) tumors. These models, developed from patients enrolled in the Lung TRACERx study, aim to provide valuable tools for studying tumor heterogeneity and therapeutic responses in NSCLC
- In June 2024, Charles River Laboratories and Oncodesign announced a collaboration to develop and commercialize humanized PDX models. This partnership aims to enhance the predictive accuracy of preclinical cancer research by integrating human immune systems into PDX models, thereby improving the evaluation of immuno-oncology therapies
- In January 2024, Champions Oncology launched the TumorGraft3D (CTG3D) platform, an innovative ex-vivo system designed to enhance the translatability of new medical entities. This platform utilizes a proprietary bank of over 1,500 patient-derived xenografts (PDXs) across approximately 50 different tumor indications, enabling more accurate preclinical evaluations of oncology therapies
- In April 2023, Charles River Laboratories introduced the NCG™ mouse model, a triple-immunodeficient mouse capable of hosting xenograft cells, tissue, and human immune system components. This model is designed to support preclinical cancer immunotherapy research by enabling the study of tumor biology and therapeutic efficacy in a humanized immune environment
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.